The FEBS Journal Open Access Round-up #2

Battling Neuroblastoma: A New Hope!
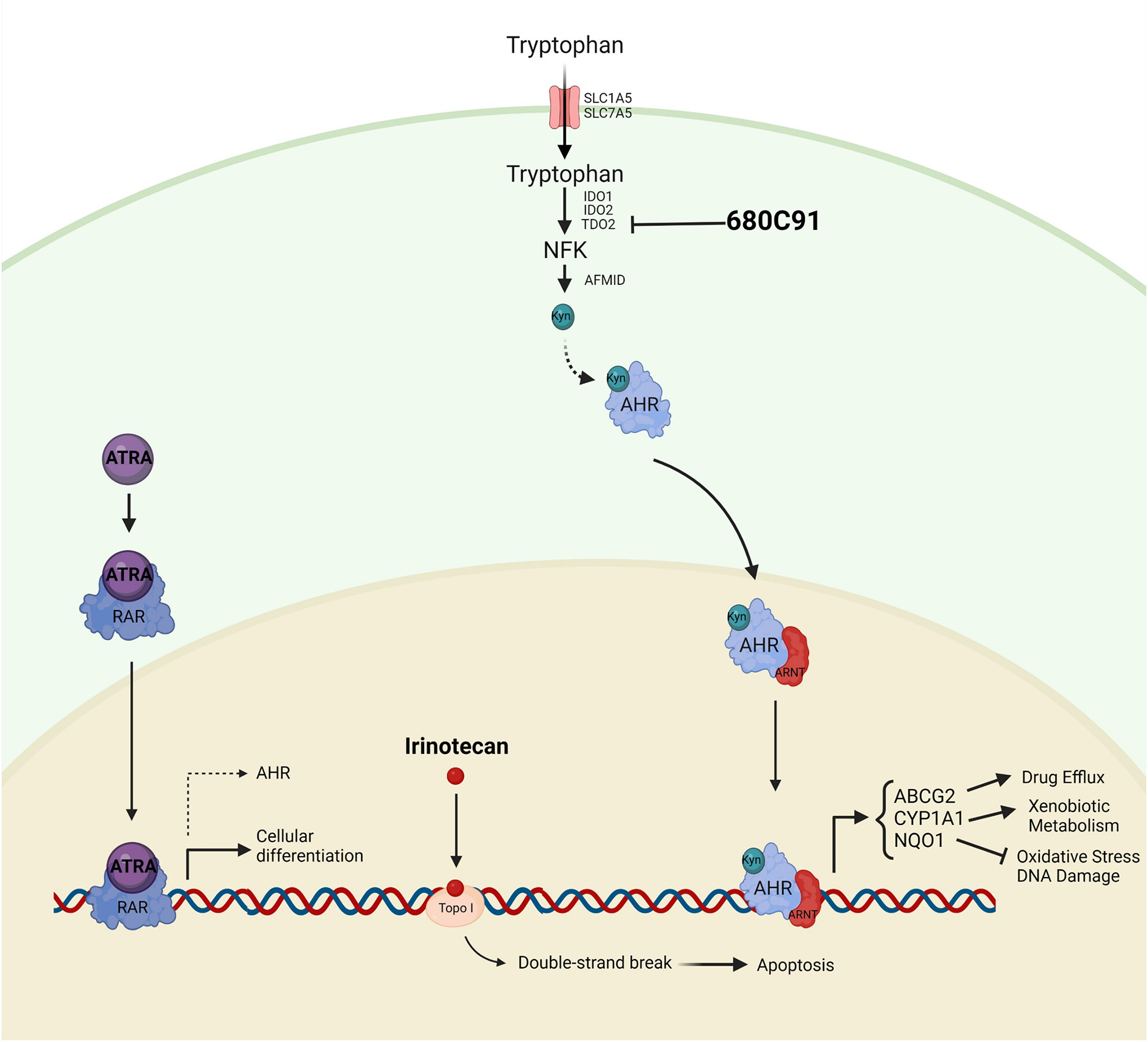
Neuroblastoma, a notorious childhood cancer, is very challenging to treat. The hunt, therefore, is on to identify new targets to aim at in the fight with this often-aggressive cancer.
In their new work, Lopes Santos et al. have investigated the role of the Kynurenine-AHR (aryl hydrocarbon receptor) pathway in neuroblastoma cells, with the hope of being able to identify a chink in the armour of the cancer’s defences. They found that AHR and its target genes (such as ABCG2, which encodes a transporter involved in drug efflux) were upregulated upon drug treatment of neuroblastoma cells. AHR is a transcription factor that, upon binding to its ligand Kynurenine translocates to the nucleus where it interacts with ARNT1 to form a transcriptionally active dimer. Importantly, Kynurenine is a product of the Trp pathway via IDO1/2 and TDO2. The authors found that neuroblastoma cells are sensitive to TDO2 inhibition, and that combining TDO2 inhibition with existing chemotherapeutic agents (ATRA or irinotecan) showed synergistic anticancer effects, revealing potential vulnerabilities. Although neuroblastoma is a highly genetically heterogeneous disease, tailoring treatment to patients with active Kynurenine-AHR pathways could improve outcomes for this high-risk subgroup of patients.
High Tension relations: Salmonella and intestinal edema
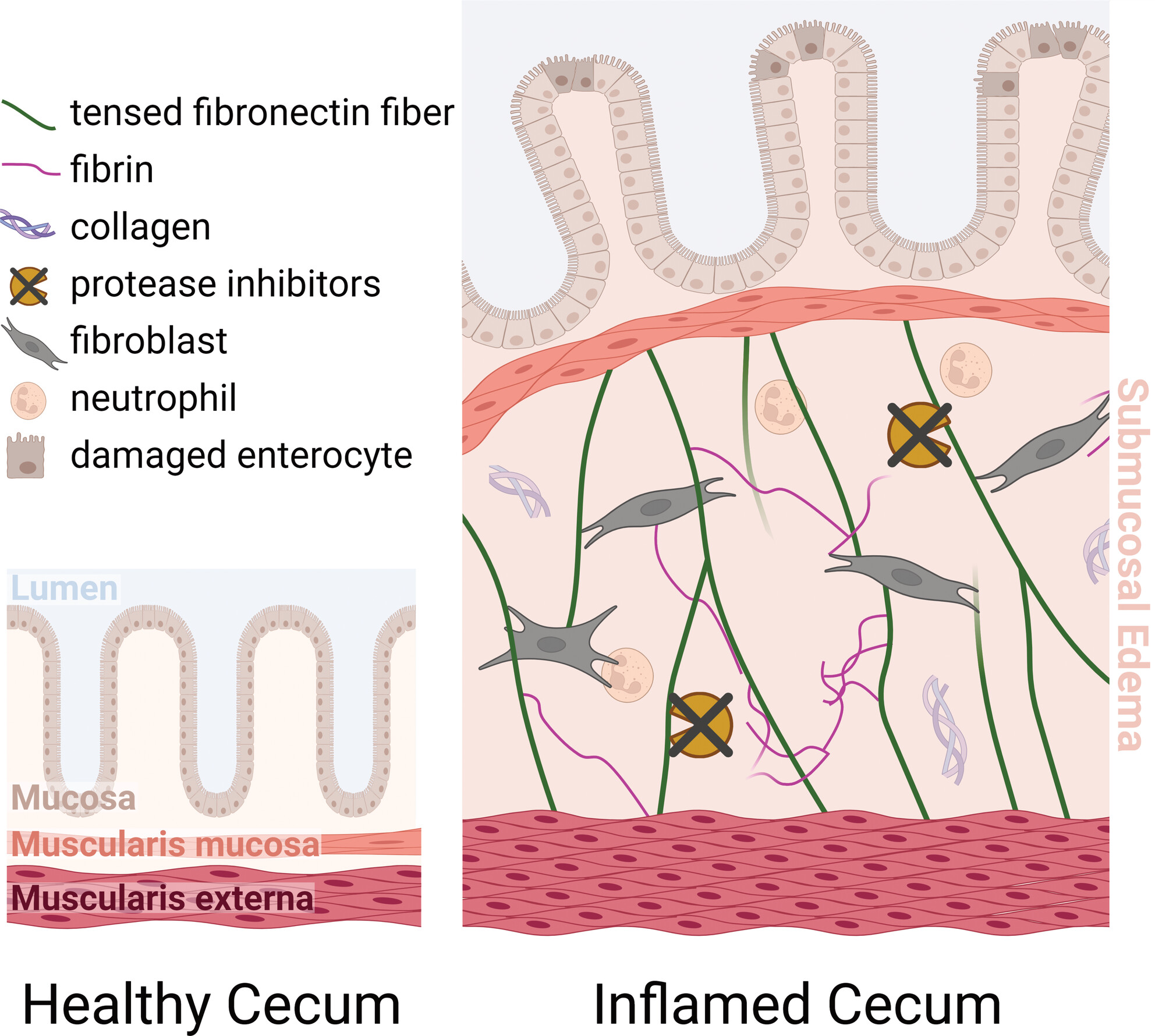
Salmonella Typhimurium (s.Tyr), is a major cause of gastroenteritis in humans. During an infection, like when consuming contaminated food, our inflammatory responses will try to limit the size and spread of the infection. This proinflammatory response in the gut leads to intestinal edema – fluid accumulation in within the interstitium of the intestinal wall tissue. This swelling reduces the gut luminal volume, limiting the colonisable space for S.Tyr. However, the forces created by this massive fluid influx are a major challenge to tissue integrity. In this impressive open access article, Roppold et al. ask which molecular mechanisms might have evolved to limit the risk of tissue integrity failure in severe infections?
Laser capture microdissection and advanced proteomics allowed the team to identify candidate proteins involved in the regulation of intestinal remodelling. They combined this with (immuno)histochemistry microscopy and the low-tension fibronectin probe, FnBPA5, to show that fibronectin, a key component of the extracellular matrix, is tensed within the edematous regions, despite the presence of proteases that could and probably should cleave it. The proteomics data also highlighted distinct mechanisms of protease inhibition to protect these stretched fibronectin fibres from degradation. They hypothesised that it was this tension in fibronectin fibres that is crucial for preserving tissue structure, effectively serving as “bungee” cords keeping the inner and outer muscle layers connected even when massive fluid influx separates them. The authors’ multidisciplinary approach is a real step forward in understanding the delicate balance between pathogen defence and tissue preservation, hopefully leading to valuable diagnostic markers for the progression of intestinal disease.
Adhesions GPCRs are sticking out in the GCPR oligomer debate
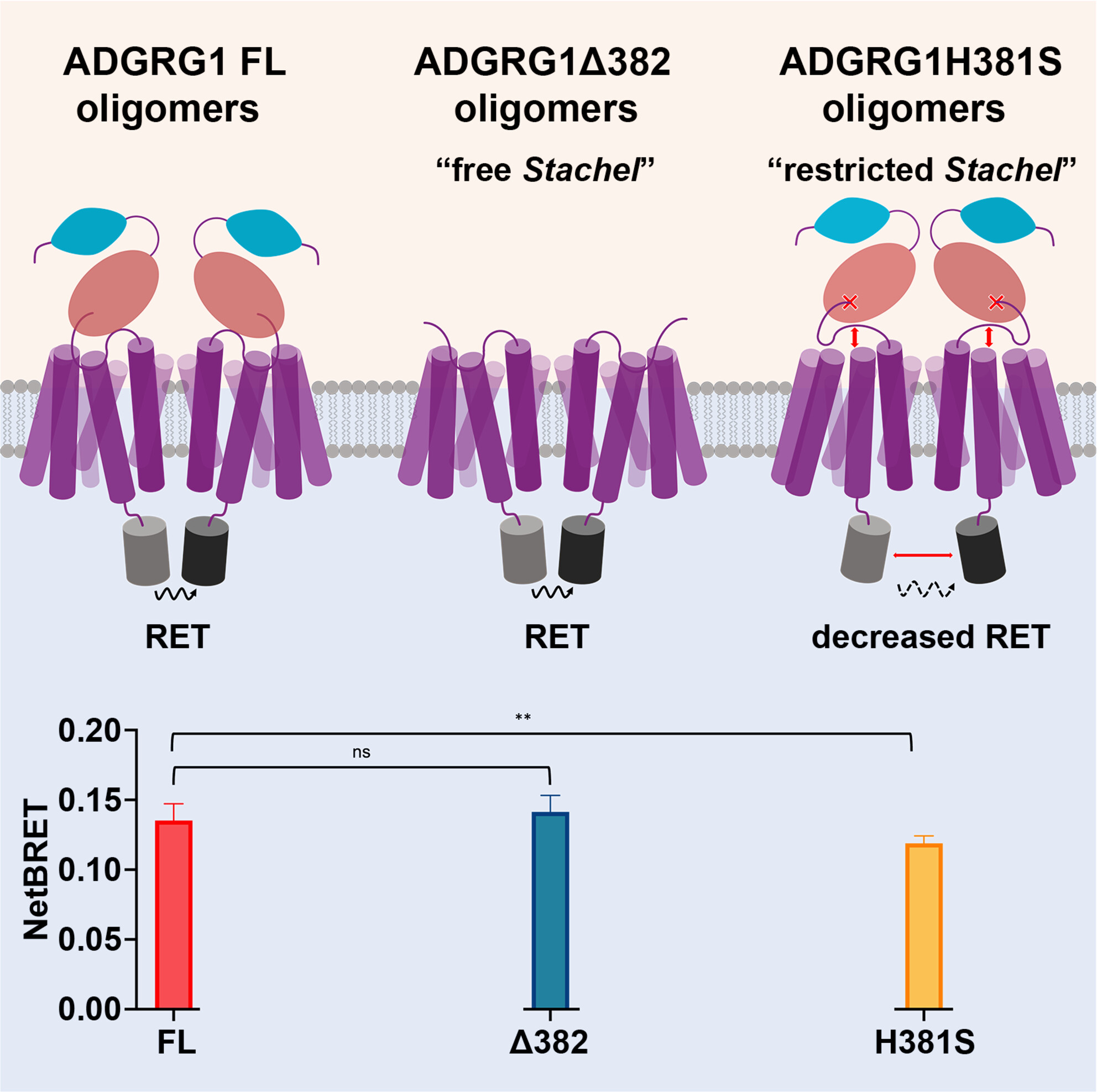
Nearly 40% of the prescribed drugs target GPCRs with the aim of treating a wealth of conditions, from Parathyroid disease and Schizophrenia to African sleeping sickness caused by Trypanosoma, the list is seemingly endless. No other fact underlines the importance of these integral 7-transmembrane (7TM) proteins, not only in a biological context but also in human health more than this. However, one of the five families of GPCRs known as adhesion GPCRs (aGPCRs) remains comparatively unexplored in terms of their potential as therapeutic targets. Therefore, a clearer understanding of aGPCR structure and signalling mechanisms in a physiological context may enable the design of drugs to treat aGPCR disfunction. GPCRs are now increasingly recognised, albeit somewhat controversially, for their ability to form oligomers, which has significant implications for their function. In this study, Cevheroğlu et al. investigated the homo-oligomerisation of the aGPCR ADGRG1 and the role of its N-terminal fragments (NTFs) . To achieve this, the authors used bioluminescence energy transfer (BRET) and Förster resonance energy transfer (FRET) in live cells to show that ADGRG1 homo-oligomerisation is driven by 7TM. Additionally, the NTF and the tethered Stachel peptide possibly contribute to the formation of these oligomeric complexes through conformation changes. This finding is pivotal, as it suggests that the Stachel motif interactions may influence the conformation of these receptor complexes, offering new insights into their functional dynamics. As ADGRG1 is linked to various human diseases, including cancer and neurological disorders, understanding its oligomerisation is therefore vital in paving the way for novel therapeutic approaches.
*The FEBS Journal is a multidisciplinary, non-profit society journal that publishes full-length high-quality papers and expert reviews relevant to the molecular, cellular and biochemical life sciences. Send us your next paper, it will be in great company!




Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.