Anne Bertolotti: "My interest in science is curiosity driven."

What drew you to your research field?
My interest in science is curiosity driven. I am keen to understand how molecules engage in dynamic processes to control life, in health and diseases. My curiosity naturally takes me to the unknown. The work we do in the lab is pluridisciplinary and data driven. As Sir Arthur Conan Doyle, Sherlock Holmes’ creator, said: “It is a capital mistake to theorize before you have all the evidence.” Science is detective work and if done with rigour and diligence, it gives us a chance to uncover how Mother Nature, through billion years of evolution, designed molecules to assemble them in the complex dynamic networks that support life.
Having uncovered components of the protein quality control systems that protect cells against proteins in bad shape, I wondered why such proteins accumulate with age, leading to devastating neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and motor neuron diseases. This then brought the idea of trying to boost these natural defence mechanisms against proteins in bad shape to enhance cellular resilience, a modality generically applicable to improve fitness in diverse diseases, including the age-related neurodegenerative diseases. Our curiosity-driven research has led to two small molecules currently tested in humans for the treatment of neurodegenerative diseases.
What do you consider the most formative phase of your research career?
A career is not one event but a series of formative events and quite a bit of serendipity. As a PhD student I had two mentors – a junior PI (Laszlo Tora) and a senior one (Pierre Chambon) – and was influenced by both in different ways. My taste for the unknown was not acquired through my PhD, but was innate. I chose to do a postdoc with an endocrinologist (David Ron) bringing biochemistry to a completely new field, thereby discovering components of what are now known as two famous pathways: the mammalian unfolded protein response and the integrated stress response. My curiosity for the unknown and aspiration to tackle challenging problems relevant to human health brought me to LMB (MRC Laboratory of Molecular Biology, Cambridge, UK), where thinking big is possible, due to long-term funding and a unique research culture.
What do you see as the most important developments in your field in the past 10 years?
The field of protein quality control is a mature field. The most significant protein quality control pathways were discovered several decades ago and this then brought the opportunity to discover small molecules to manipulate them for potential therapeutic benefits. The range of diseases that can benefit from manipulations of protein quality control systems is huge. Although there are only a small number of approaches and molecules that have reached clinical stage, there will be more in the coming years. I expect that these developments will be transformative for human health in the next decade or so.
What’s exciting or challenging in your research area right now?
We have recently discovered that inhibitors of one kinase of the integrated stress response can actually activate a sister kinase and thereby activate the pathway they were destined to inhibit. Efficacy for these inhibitors has been reported in a number of disease models. Our work suggests that efficacy in these studies is driven by activation rather than inhibition of the pathway. This completely changes the way we think about these inhibitors and their kinases. We are now pursuing these findings, hoping to understand the underlying molecular mechanism driving activation, the therapeutic potential of kinase activation in diseases and to generate new molecules. This line of work came by surprise. We couldn’t predict this!
What’s currently your favourite technique?
I have no favourite technique! Our work is pluridisciplinary by nature, combining chemistry, biochemistry, biophysics, structural biology and cell biology with mouse models of diseases. We do whatever it takes to answer scientific questions.
What aspects of your life as a researcher do you most enjoy?
I enjoy the freedom to pursue whatever line of work I choose. Freedom has a price… I love mentoring young scientists, on a day-to-day basis. One needs to enjoy small achievements. A conclusive experiment is priceless and the intellectual process that guides the design of the killer experiment (the experiment that tells us to stop or continue a line of research) is a lot of fun! It is hugely rewarding to guide young scientists to grow scientifically and often personally as well. I am proud to have inspired trainees to start their own lab, join biotech companies as well as become editors. The lab isn’t “one size fits all” but a place where I try to identify the unique strength of young scientists and help them develop wherever they thrive. Above all, it needs to be fun. The lab is a playground for new ideas and discovering something no one has seen before is tremendously exciting. As joint Head of the Neurobiology Division, I try to foster a positive and supportive research culture and encourage people to think big. In scientific panels and review committees, I enjoy encouraging an unbiased and data-driven attitude.
How can research scientists find a reasonable work–life balance?
I work on cellular homeostasis and finding a reasonable work–life balance follows the same principle. We have to adapt to conflicting demands on our time and learn to make mutually exclusive choices. There is a lot of demand on our time and we can’t do it all. I try to choose what I do based on where I think I can make a unique and significant contribution. Science is competitive and requires long hours of focused work, but I don’t believe one can achieve much when unhappy or unfit. I often solve problems at the gym or in my garden or whilst cooking! A critical preparation to a significant talk for me is an intense workout. I think I inspire people around me to find what works for them.
Introductory videos:
https://www.ibiology.org/speakers/anne-bertolotti/
Two recent papers:
Szaruga, M. et al. (2023) Activation of the integrated stress response by inhibitors of its kinases. Nat. Commun. 14, 5535. https://doi.org/10.1038/s41467-023-40823-8
Fatalska, A. et al. (2024) Recruitment of trimeric eIF2 by phosphatase non-catalytic subunit PPP1R15B. Mol. Cell 84, 506–521.e11. https://doi.org/10.1016/j.molcel.2023.12.011
More information on the IUBMB Jubilee Award Lecture at the 49th FEBS Congress
Anne Bertolotti will deliver the IUBMB Jubilee Award Lecture at the 49th FEBS Congress in Istanbul, Türkiye on Wednesday 9th July 2025 on ‘Boosting protein quality control in neurodegenerative diseases: From the bench to the clinic’: 2025.febscongress.org/
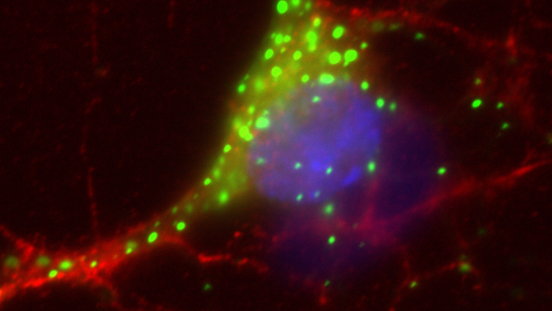
Top image of post: A neuron expressing mutant Huntingtin, forming green aggregates. Credit: Erwann Rousseau.





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.