
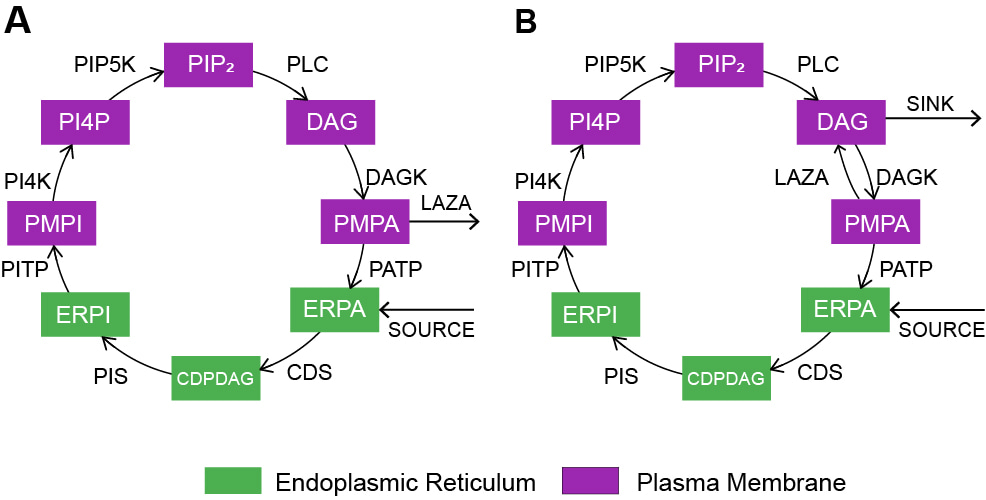
Evidence of sinks and sources in the phospholipase C-activated PIP2 cycle
Krishna, S. and colleagues, FEBS Letters
Phosphatidylinositol 4,5 bisphosphate (PIP2) can act as second messenger in eukaryotic signalling cascades upon hydrolysis by receptor-mediated phospholipase C (PLC) activity. The PIP2 cycle, essential to regenerate PIP2 and to sustain PLC signalling, is subject of studies since decades. Through mathematical models, the authors show that the PIP2 cycle may contain at least two unknown steps funnelling metabolic intermediates, offering a new perspective to experimental interpretation.
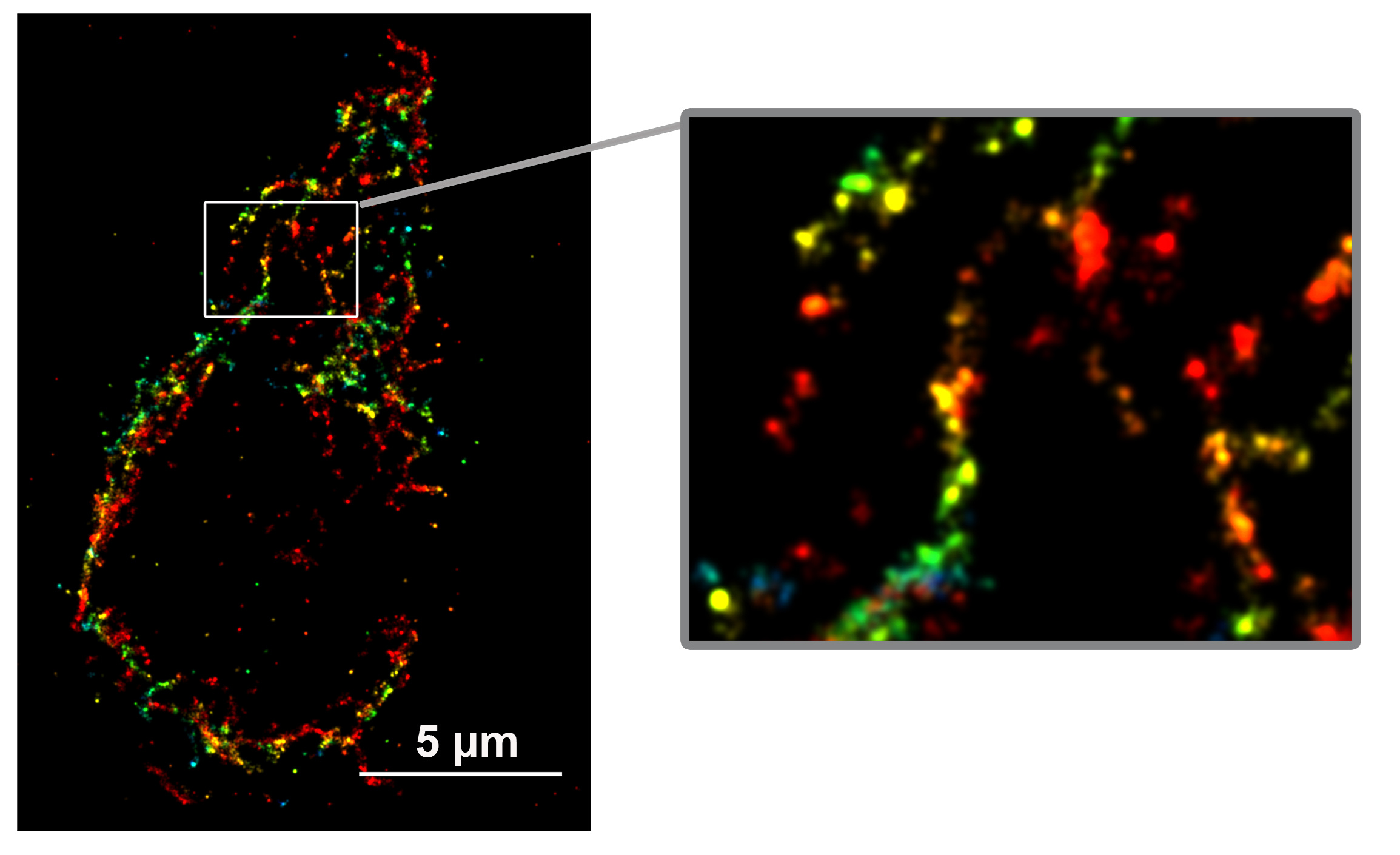
Regulation of glucose-stimulated insulin secretion by ATPase Inhibitory Factor 1 (IF1)
Dlaskova, A. and colleagues, FEBS Letters
The ATP synthase inhibitory factor 1 (IF1) has been reported to bind ATP synthase under low pH conditions and inhibit its activity. Here, the authors find that IF1 is expressed in pancreatic b-cell mitochondria and interacts with ATP synthase under normal physiological conditions. Silencing IF1 in pancreatic b-cells upregulates cellular ATP levels and increases insulin secretion over a range of glucose concentrations. Thus, IF1 regulates glucose-stimulated insulin secretion in pancreatic b-cells and may be a drug target for type 2 diabetes.
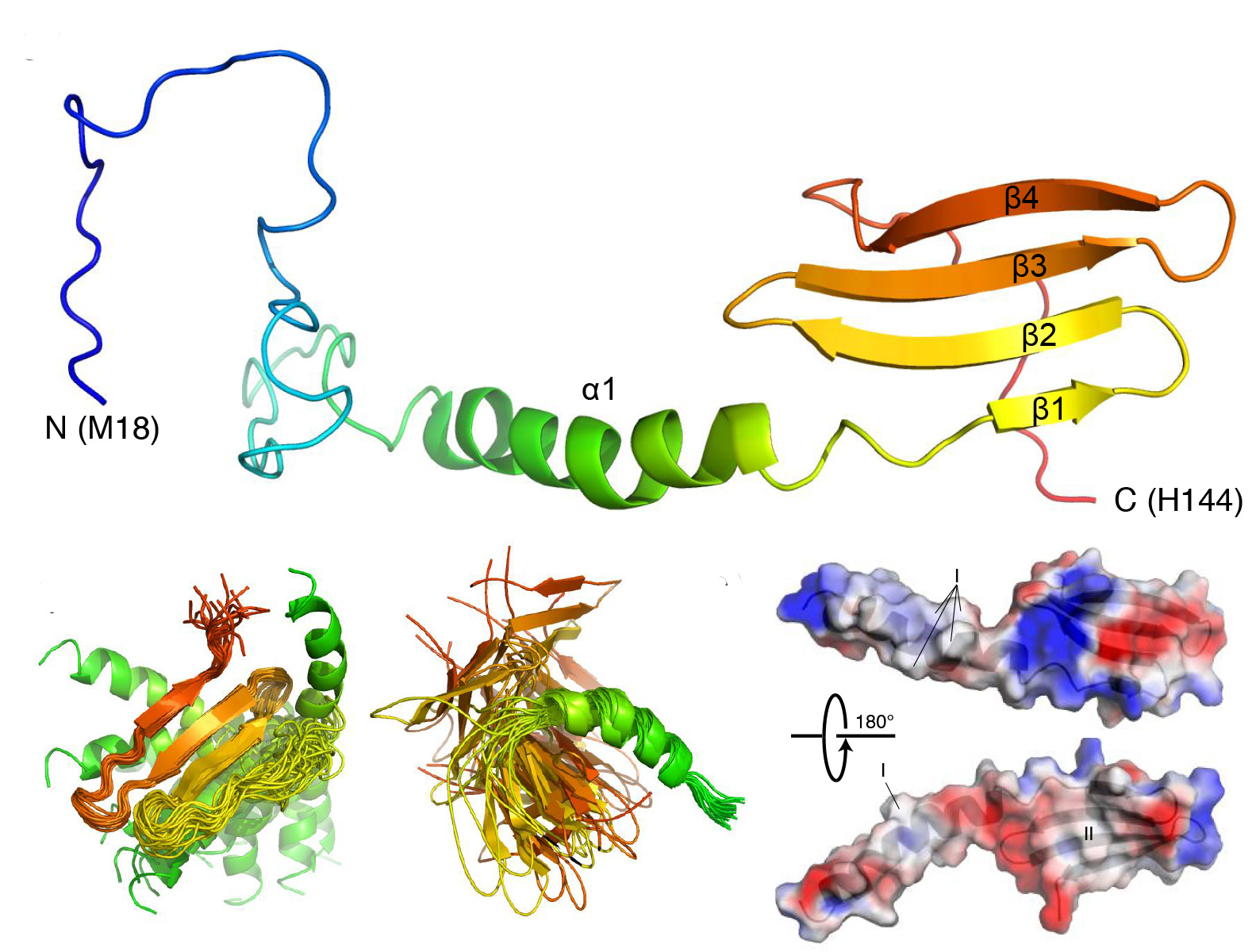
Structural and functional characterization of the Curli adaptor protein CsgF
Ritter, C. and colleagues, FEBS Letters
The fibrillar curli proteins produced by enteric bacteria such as E. coli are involved in cell adhesion and biofilm formation. Their ability to aggregate into functional amyloids makes them paradigms for understanding amyloidogenesis in neurodegenerative diseases such as Alzheimer’s and Parkinson’s. CsgF is a cell surface protein required for the attachment and assembly of curli amyloids. Here, the in vitro characterization and solution NMR structure of CsgF provide insights into its protein-protein interactions and function in vivo.


Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.