The FEBS Journal Open Access Round-up #3

We highlight two articles for you this month, as one of us is off to swap the cold and wet weather in the north of England for the warm sunshine of the Mediterranean for a week. But if you are still stuck somewhere cloudy and chilly, fear not, you can bask in the warmth of some fantastic open access science published in the FEBS journal!
Cut and Run…..away with an attached protease.
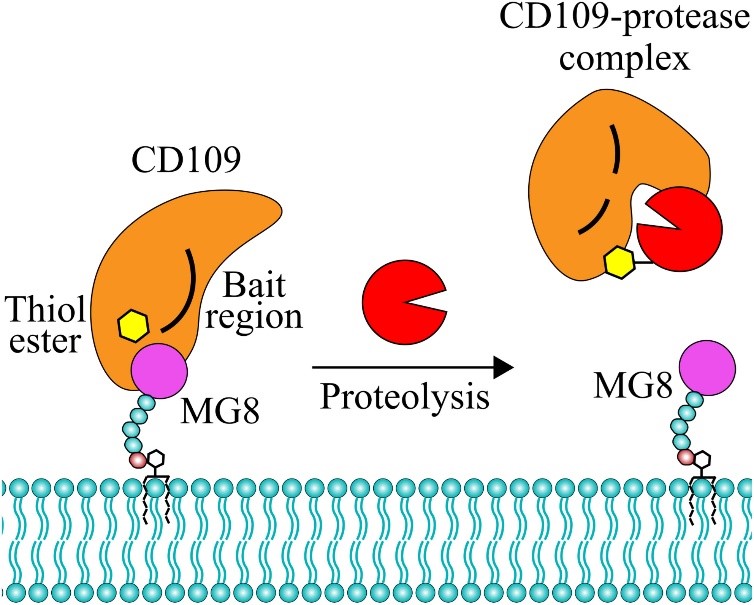
First up, we have an exciting study by Jensen et al. on CD109, a transforming growth factor (TGF)-β co-receptor. CD109 regulates TGF-β receptor endocytosis and degradation and has a key role in underlying oncogenic signalling. CD109 is a member of the alpha-macroglobulin protein (A2MF) superfamily known for undergoing conformational changes upon protease interaction. However, CD109 has several features that are unique among A2MF proteins that makes it hard to predict the role of its thiol ester, whether it can inhibit proteases, and the functional consequences of its putative conformational change. To further characterise CD109 as an A2MF protein, Jensen and colleagues produced soluble recombinant CD109 by removing its GPI motif. They then attacked this soluble CD109 with most proteases you can think of, and a couple you can’t – 16 in total. Twelve of these preferentially cleaved CD109 within its predicted bait region, causing a thiol ester-exposing conformational change. This conformational change releases the non-covalently associated MG8 domain of CD109, which liberates it from the cell membrane. Although CD109 role as a TGF-β co-receptor is well established, the implications of its conformational state have not previously been considered. As such, the downstream effects of the changes described in this paper remain to be studied. It is very likely that the conformational state of CD109 governs its interactions with other proteins, and this research opens the proverbial door to exciting new functional discoveries in the TGF-β signalling field.
A new way to cause a STING for cancer
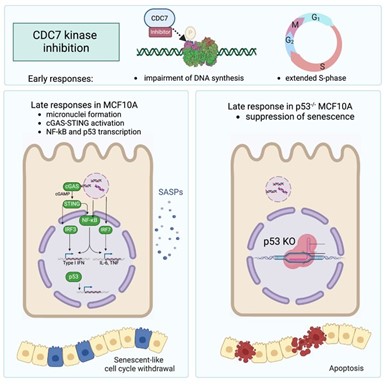
Next, we dive deep inside the cell to focus on Cell division cycle 7-related protein kinase (CDC7). This serine-threonine kinase is a key regulator of DNA replication and of the DNA damage response. Its inhibition specifically impedes the restarting of paused replication forks and decreases the efficiency of origin firing. With such a significant role in DNA replication, it is no surprise that numerous small molecules targeting CDC7 have been developed as potential cancer therapeutics. This study from lead authors Cazzaniga, Göder and Rainey explores the long-term effects of inhibiting CDC7 by two widely used inhibitors (XL413 and TAK-931). The authors found that both CDC7 inhibitors lead to a senescent-like state and to the formation of micronucleated cells, which rupture, activating the GAS-STING pathway. They postulate that GAS-STING signalling could be linked to the expression of many inflammatory genes, altering the tumour microenvironment. Importantly, the authors found that a cell’s fate post-CDC7 inhibition hinges on the presence of p53, a tumour suppressor protein. Cells with functional p53 enter senescence, while p53-deficient cells are directed towards apoptosis. This is a big finding for cancer therapy development, because by driving cancer cells into senescence, they can be further targeted with senolytic agents. This offers a new really cool avenue for cancer treatment strategies.
*The FEBS Journal is a multidisciplinary, non-profit society journal that publishes full-length high-quality papers and expert reviews relevant to the molecular, cellular and biochemical life sciences. Send us your next paper, it will be in great company!





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.