Shedding light on key genes behind aggressive phenotype of pleural mesothelioma
Pleural mesothelioma (PM) is a rare cancer with an increasing mortality rate due to its aggressive character and challenges in clinical management. Challenges in the clinic vary, as some patients are diagnosed later, some do not respond to the treatment regimen, and some patients face relapse after chemotherapy (1). Extensive exposure to asbestos fibres are the main causes of the disease and these could be occupational (exposure at workplaces like mines, automobile, or cement industry), domestic (exposure to asbestos fibre-contaminated food or water in homes or water pipes), or environmental (exposure from the natural environment like soil or rock) (2). Until recently, asbestos was commonly used. The long latency period from asbestos inhalation to symptom appearance is also a factor accounting for the increased incidence rates of PM.
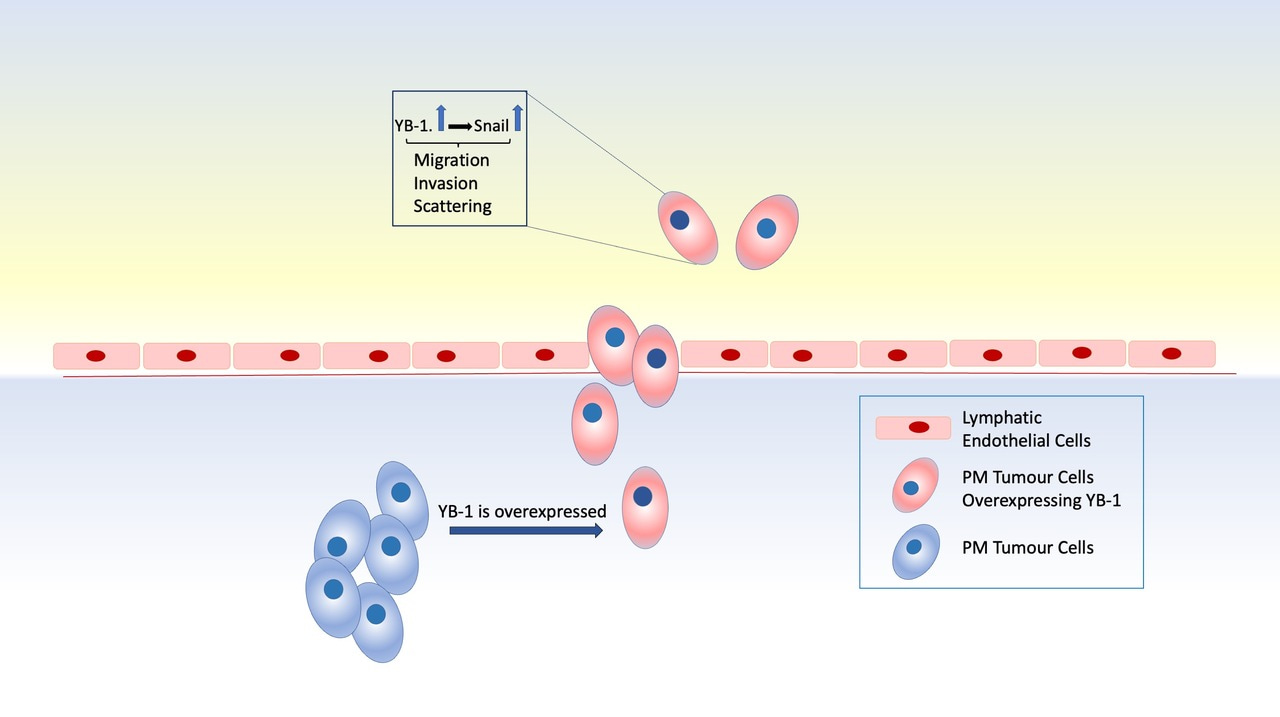
Since recurrence is a major challenge in PM treatment, efforts in understanding the molecular mechanisms behind PM's aggressiveness are vastly important. In the current issue of Molecular Oncology, Schelch et al. (3) focused on the role of the oncoprotein YB-1 in PM cell migration. YB-1 is a nucleic acid binding protein which has been shown to be overexpressed in many cancers. YB-1 has been shown to exert its effects on target gene expression via transcriptional and post-transcriptional mechanisms.
Schelch et al. showed that stable overexpression of YB-1 in PM cells results in an altered cell cycle, extending M phase duration but shortening the doubling time and increasing scattering and colony formation abilities of PM cells. Wound healing and cell tracking experiments revealed that YB-1 overexpression renders the cells more motile. The migratory capacity of PM cells was further tested in a fluorescently labelled and doxycycline-inducible YB-1 overexpressing cell line injected into a zebrafish xenograft model. Fluorescently labelled YB-1 overexpressing cells were injected into the perivitelline space of the 48h old zebrafish larvae. When the cells were tracked in the tail, the migrating cell number was found to be higher in the group of cells overexpressing YB-1 compared to the control. Additionally, the effect of YB-1 on the intravasation capacity of PM cells was investigated via a co-culture intravasation assay. In this experiment, tumour spheroids were placed on endothelial cells and the intravasation of tumour cells in the lymphatic endothelial layer was higher when YB-1 was overexpressed. Rescue experiments using YB-1 RNAi further confirmed the role of YB-1 in promoting PM cell motility.
Authors also shed light into the mechanism behind YB-1 in PM motility by investigating gene expression patterns of several stem cell markers and motility-related genes. In these experiments, the expression of EGFR and Snail were controlled by YB-1. Snail was further found to be regulated at the post-transcriptional level, whereas EGFR was suggested to be regulated at both the transcriptional and post-transcriptional level. RNAi-mediated silencing of EGFR and the use of a specific EGFR inhibitor, erlotinib, resulted in diminished PM migration which could be rescued by YB-1 overexpression. Moreover, when YB-1 was overexpressed concurrently with Snail knockdown, a change in cell migration was not observed suggesting a novel role for Snail in YB-1-mediated migration of PM cells.
Conclusively, the findings of Schelch et al. highlight the pivotal role of YB-1 in PM cell migration through illuminating a link between YB-1 and Snail. The researchers also identified a role for Snail-dependent YB-1 induced PM migration. Authors also revealed for the first time that YB-1 related PM cell migration is controlled through post-transcriptional regulation of Snail. The potential prognostic value of YB-1 in PM makes it a great target in the treatment of this rare disease and studies revealing mechanisms behind important regulators like YB-1 are of great importance.
References
- Scherpereel A., Wallyn F., Albelda S.M., Munck C. (2018), Novel therapies for malignant pleural mesothelioma. Lancet Oncol. doi: 10.1016/S1470-2045(18)30100-1
- Peña-Castro M., Montero-Acosta M., Saba M. (2023), A critical review of asbestos concentrations in water and air, according to exposure sources. Heliyon. doi: 10.1016/j.heliyon.2023.e15730
- Schelch K., Eder S., Zitta B., Phimmachanh M. Johnson T.G., Emminger D., Wenninger-Weinzierl A., Sturtzel C., Poplimont H., Ries A., Hoetzenecker K., Hoda M.A., Berger W., Distel M., Dome B., Reid G., Grusch M. (2024), YB-1 regulates mesothelioma cell migration via Snail but not EGFR, MMP1, EPHA5 or PARK2. Mol Oncol. doi: 10.1002/1878-0261.13367
Image by Molecular Oncology





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.