Reimagining enzymes for synthetic biology with AI
This article was written by Laurynas Karpus, CEO of Biomatter, and was originally published on the SynBioBeta website on 1 May 2024.
The growing demand for new enzymes is catalyzing the evolution of industrial biotechnology, biopharmaceuticals, bioremediation, and R&D while simultaneously driving the transformation to sustainable manufacturing processes.
As the world’s population continues to grow, there is an urgent need for enzyme-based solutions that can redefine manufacturing and provide healthy, nutritious food more efficiently with lower environmental impact. These demands have spurred researchers and companies to push the boundaries beyond what was once thought possible with enzyme engineering.
Reimagining enzyme design
Until recently, the strategy for developing industrial enzymes relied on identifying those found in nature and enhancing them via incremental improvements through protein engineering. This approach, however, results in “Frankenstein” enzymes that are not always suited for the demands of industrial processes.
To transform industries and meet global sustainability goals, we must transcend the limits of working with natural enzymes alone and redesign the fundamental structures and functions of enzymes, rewriting nature’s catalytic tools for yet-to-be-imagined applications.
Biomatter has reimagined enzyme design in response to these complexities, taking a radically different approach: starting with the ideal end state in mind. That means defining clear objectives, requirements, constraints, and acceptance criteria. It means designing enzymes that exhibit precise function and performance characteristics—the specific chemistries and functions tailored to the specific applications. This shift from identifying and modifying to a de novo, ground-up design approach marks a pivotal change in biotechnological innovation.
Pioneering generative AI enzyme design
Starting in 2019, Biomatter pioneered the use of generative AI to expand the protein sequence space by developing ProteinGAN, a specialized variant of the generative adversarial network that can learn natural protein sequence diversity and enable the generation of functional protein sequences.
Since then, Biomatter has perfected proprietary generative AI tools and physics engines to design enzymes from the ground up, moving beyond the constraints of nature's blueprints.
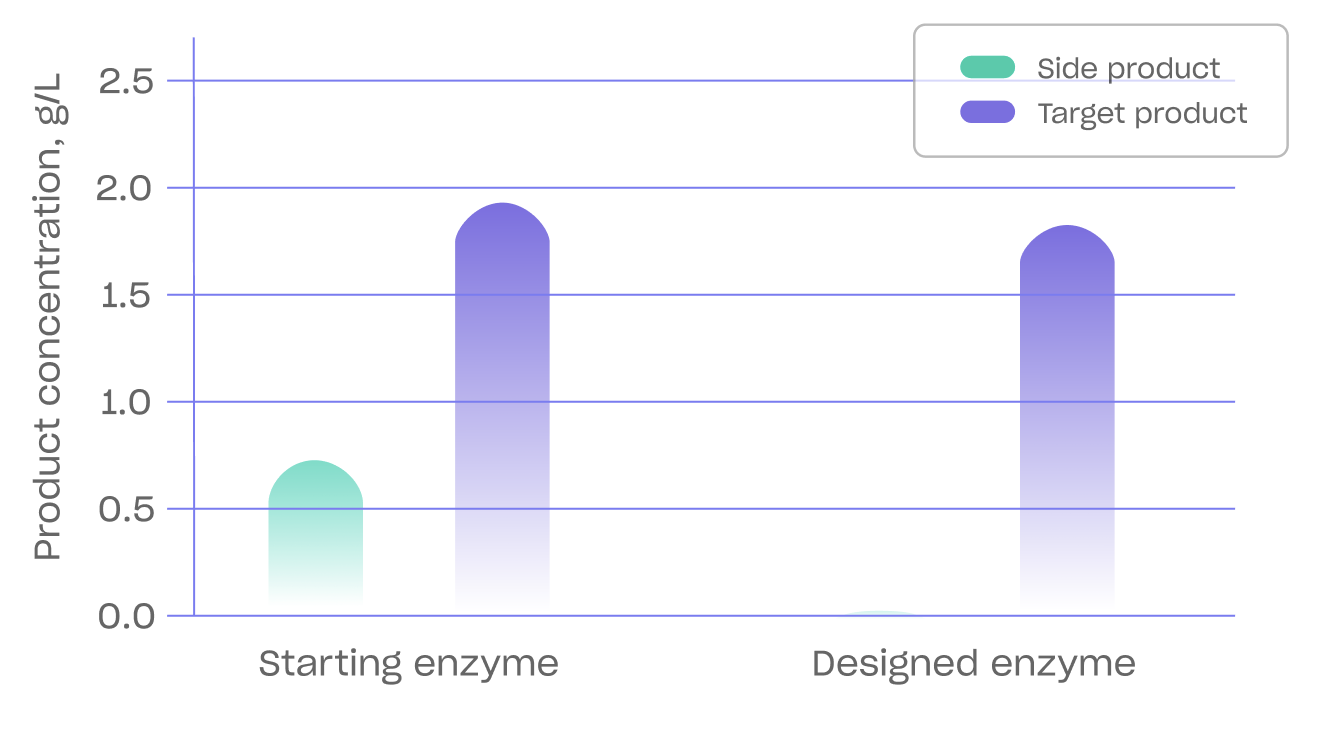
An example of Biomatter’s enzyme engineering and precision fermentation prowess is its recent collaboration with Kirin Holdings to increase the production of Lacto-N-fucopentaose (LNFP I), the second most abundant fucosylated human milk oligosaccharide (HMO) in breast milk after 2'-Fucosyllactose (2'-FL). Until now, producing LNFP I at a high titer without producing 2'-FL has been a challenge.
LNFP I is produced through an enzymatic reaction catalyzed by the enzyme α1,2-fucosyltransferase (α1,2-FucT).
Biomatter redesigned the substrate binding pocket of α1,2-fucosyltransferase (FsFucT) and the hydrophobic residues on the enzyme's surface. The resulting enzyme selectively produced LNFP I compared to 2'-fucosyllactose (2'-FL) and was optimized for batch-fed fermentation.
As a result of this work, the strain expressing the newly redesigned FsFucT enzyme showed increased accumulation of LNFP I and only produced a trace amount of 2'-FL. The ability to decrease the amount of by-products is essential when manufacturing on an industrial scale.
Addressing perceived risks
While embracing AI for enzyme design offers unprecedented potential, it also introduces certain perceived risks and limitations that merit consideration. These include:
- The quality and availability of data used for training AI models could pose challenges, potentially affecting the accuracy and reliability of engineered enzymes,
- An incomplete understanding of the complex structure-function relationship of AI-designed enzymes – that is, AI’s inability to tackle complex enzyme engineering problems (i.e., increasing enantioselectivity) or very complex manipulations (i.e., backbone and active pocket design, functionalizing previously nonfunctional protein scaffolds, or creating new functions),
- The lack of experimental validation that can lead to uncertainties about an enzyme’s functionality under real-world conditions.
It is important to recognize these considerations not as insurmountable obstacles but as integral aspects of the current innovation landscape in synthetic biology. These challenges can be overcome by supporting AI with physics and empirical scientific inquiry to refine and validate designs.
Biomatter uses fully experimentally validated AI and physics-based software tools to address the above challenges. It showcases that designed enzymes can be developed, tested, and produced quickly. Our methodology erases the boundaries between the conceivable and the achievable, the possible and the impossible.
Biomatter's enzyme design capabilities enable near-perfect control over enzyme design, making previously impossible technical jumps possible while opening the door to unique enzyme-based products and technologies. The company develops tailor-made enzymes with unique functions that are fully optimized for industrial applications by increasing activity, specificity, stability, and expression levels by functionalizing nonfunctional scaffolds and discovering new proteins on accelerated timelines.
We are on the cusp of revolutionizing protein engineering, entering an era where researchers are not constrained by nature's initial designs but can create bespoke, fit-for-purpose enzymes.
Top image by Biomatter.





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.