New European Partnership for Personalised Medicine (EP PerMed)

The newly launched European Partnership for Personalised Medicine (EP PerMed) aims to advance future healthcare for all citizens through personalised therapy, diagnosis and prevention strategies. With a total budget of around €375 million, it will provide significant support not only for the transnational development of personalised medicine approaches, but also for their successful translation into clinical practice. European Partnerships are initiatives in which the European Commission and private and/or public partners commit themselves to jointly support the development and implementation of a research and innovation program. They make a significant contribution to achieving the EU's political priorities. EP PerMed brings together more than 50 ministries, funding organisations, research institutes from 24 countries and 10 European regions as well as European research infrastructures (e.g., BBMRI-ERIC) and other partners active in the innovation field (e.g. EIT Health). The partnership builds on the results and experience of several international initiatives and projects in the field of personalised medicine, as well as more than 15 years of successful collaboration among many of the partners involved. Over the next ten years, with most of EP PerMed’s budget multidisciplinary consortia will be funded. Other activities aim to support the innovation steps and the implementation of personalised medicine approaches on the basis of research achievements.

Joint Transnational Calls for Research Proposals
The first EP PerMed Joint Transnational Call (JTC) 2024 ‘Identification or Validation of Targets for Personalised Medicine Approaches (PMTargets)’ was published on 2 January 2024. In total, 38 funding organisations participate in the call with an available budget of over 45 Mio€. First projects are expected to start by the end of 2024.
A total of seven annual Joint Transnational Calls are foreseen during the lifetime of EP PerMed. The next one is expected to be launched in early 2025. As outlined in the Strategic Research & Innovation Agenda for Personalised Medicine (SRIA PerMed), additional activities will focus on actions to support and accelerate personalised medicine innovations and their uptake by healthcare systems. These include, for example, partnering and networking events between research communities and the private sector, personalised medicine pilots, particularly in a regional context, and surveys to identify and contact key stakeholders in healthcare systems and medical societies.
Broad Scope and Activities beyond Joint Calls
To support the implementation of personalised medicine, EP PerMed will continuously map and communicate progress and knowledge in all relevant areas to demonstrate the value of personalised medicine approaches in terms of quality of care, quality of life, socio-economic impact and sustainability. This includes knowledge about new therapies, biomarkers, diagnostics, devices, preventive measures, and processes, as well as insights relating to ethical, legal and social implications (ELSI) and policy aspects to support the decision-making processes and the citizen engagement, but also the acceptance by end-users such as healthcare professionals and the society. Awareness, engagement, education, training, and information programmes targeting patients, healthcare professionals, researchers and citizens will be developed and supported.
Two complementary approaches implemented in EP PerMed, the so-called Radar and Accelerator, will aim for fast-track development, innovation and uptake of personalised medicine approaches. Specifically, the partnership will foster communication and cooperation between public and private partners through dedicated funding activities (innovation, demonstration and validation projects) and support for education and training or synergy activities, including summer schools, hackathons, matchmaking events or business incubation programmes.
Strategic Research & Innovation Agenda for Personalised Medicine
Consistent with an impact-driven approach and to support personalised medicine, a Strategic Research and Innovation Agenda for Personalised Medicine was developed already before the partnership was fully set up. This process was strongly supported by the International Consortium for Personalised Medicine (ICPerMed) and related EU-funded coordination and support actions as well as ERA PerMed and other European and national initiatives.
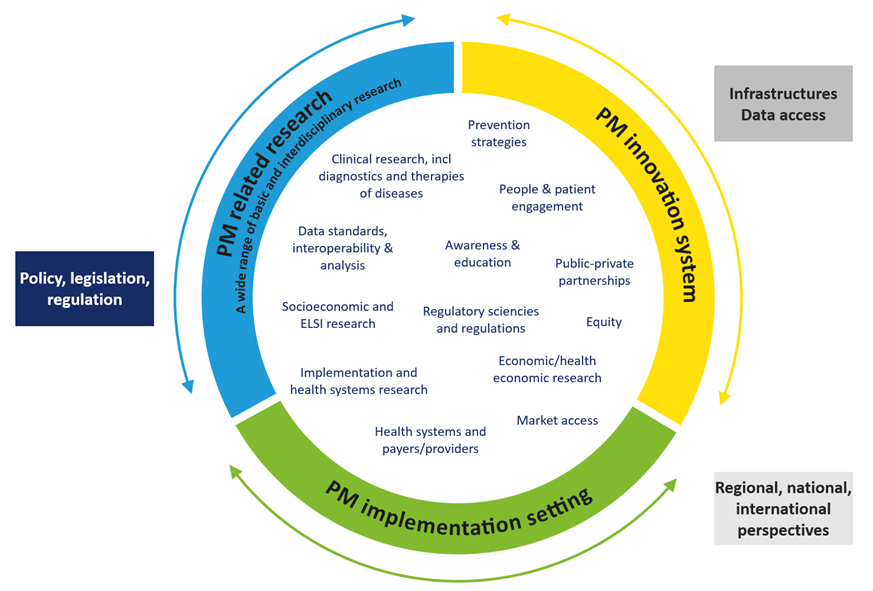
The SRIA aims to ensure that the long-term vision of EP PerMed is translated into tailored roadmaps with funding and supporting activities, objectives and measurable outcomes. Interviews were conducted with more than 70 experts and stakeholders to gather general ideas, recommendations, and information related to PM and, in particular, to identify so called “Triplets of Action, ToA”, defining specific challenges, objectives, and the expected outcome of actions to foster research and implementation of innovative personalised medicine approaches.
If you are interested in EP PerMed’s activities, subscribe to their newsletter or follow them on Twitter or LinkedIn.

All images are from EP PerMed.





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.