Multidisciplinary collaborative research to develop Organ-on-Chips in the UK
Organ-on-Chips (OOCs) are poised to transform the drug discovery pipeline, as well as biomedical research. These cellularised, microfabricated, microfluidic devices mimic the structure and function of human organs, offering a highly controlled environment for studying physiological processes and disease mechanisms.
One of the most pressing challenges in drug development is the ability to accurately predict the safety and efficacy of novel therapeutics, in a human context. Traditional preclinical models, such as animal testing and 2D cell cultures, often fail to accurately reflect human physiology and biological responses, contributing to a persistent attrition of the drug discovery pipeline. OOCs address this challenge by providing a more physiologically relevant platform for drug testing, allowing researchers to study complex cellular interactions and responses in a controlled environment that can more accurately capture human biology.
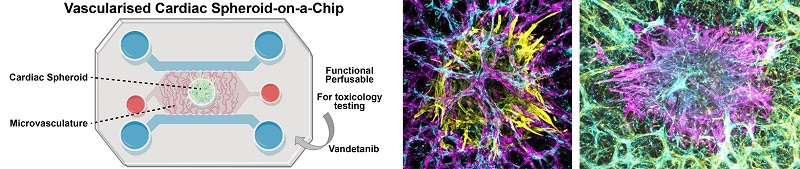
This project, funded by the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs), focused on translating microvascularised chips developed to host target tissue models, for the testing of cardiotoxicity. The collaborative effort between the laboratory of Professor Gautrot at Queen Mary University London (QMUL), and Medicines Discovery Catapult (MDC) combined complementary academic and industrial expertise, to investigate microfabricated microvascularised cardiac spheroid models for safety testing.
The MDC is an independent, not-for-profit innovation centre for drug discovery, reshaping drug discovery for patient benefit. It supports drug discovery innovators by making world-class expertise, facilities, complex technologies and advanced analytics accessible to enable successful medicines discovery.
The development of OOCs is increasingly seen as critical to reduce the reliance on animal testing in biomedical research, whilst improving accuracy for safety and efficacy prediction. The NC3Rs has been at the forefront for promoting alternative methods to animal testing and research, emphasising the importance of implementing ethical, validated alternatives.
An optimal collaboration
By bringing together expertise from the Gautrot Laboratory at QMUL in the engineering of microvascularised microfluidic chips, and that of MDC for the formation of functional cardiac spheroids, this project facilitated rapid progress towards the design and translation of microvascularised cardiac models. The resulting chips not only maintain the beating of cardiac spheroids in a hydrogel for extended periods of time, but also allow perfusion through a microvasculature, to enable the mimicking of systemic delivery.
The Gautrot Laboratory also benefited from state-of-the-art facilities for the microfabrication of advanced in vitro models, the engineering of complex multi-cellular tissues and their assaying and imaging with fluorescence microscopy, and live imaging platforms available through the Centre for Predictive in Vitro Models at QMUL.
The development of this complex OOC model required the tight coordination of microfabrication and microvascularisation efforts at QMUL and cardiac spheroid assembly and transfer at MDC. Combining these established models, exercising the specialist skills of each laboratory, was efficient and synergistic, increasing the application and technology readiness level of both approaches.
In conclusion, OOCs present unique opportunities to redesign the drug discovery pipeline, increase success rates and reduce our reliance on animal testing. This project highlights the importance of collaborative research and innovative technology in developing complex advanced in vitro models. To enable the translation of these platforms for drug discovery, multidisciplinary facilities and approaches are required.
Top image by Stefania Di Cio, QMUL.





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.