Fantastic Beasts and Why to Study Them

Sometimes, even scientists can be guilty of not seeing reality for their own assumptions. The first edition of Encyclopaedia Britannica, for example, argued that it would have been fairly easy to fit all the animals into a Noah’s ark of Biblical proportions. The reason? “If we come to a calculation, the number of species of animals will be found much less than is generally imagined… Zoologists usually reckon but an hundred and seventy species in all.”[1]
If that were true, I would have already encountered about a third of the world’s animals on my daily commute through the forests of Heidelberg to the European Molecular Biology Laboratory (EMBL). Clearly, the authors should not have assumed that a religious text intended to convey moral principles also necessarily contains exact historical and scientific information. Thankfully, old assumptions are curable by new evidence. By the time Britannica was first published, Carl Linnaeus was already overseeing the 12th edition of his Systema Naturae – a work which expanded from a few pages to several volumes, cataloguing and describing countless species. Suddenly, naturalist’s eyes were opened to the enormous diversity of organisms. Museums found themselves in a deluge of specimens from around the world, each needing classification and description – an effort that continues to the present day…
As a developmental biologist, rather than marvelling at these “endless forms most beautiful”,[2] I focus on the cellular and molecular processes that make them so. It might be natural to think that the astonishing diversity of animal bodies is generated by an equally diverse zoo of developmental processes. However, since all animals share a common ancestor and evolution tends to reuse successful solutions, the developmental toolkit is surprisingly limited. Recurring themes such as tissue folding, stretching, lumen formation, and organizing centers appear again and again across animals. A similar unity shows up at the molecular level. It is not as some people once postulated that a majority of genes in a genome are clade-specific, or that different sets of genes are used to make adults and larvae.[3] The genome is a piano capable of playing both jazz and Bach.[4]
I am not arguing that genetic and developmental innovations unique to certain animals do not exist, but rather that the foundational developmental toolkit is strikingly similar across species. To grasp these conserved principles, it should be sufficient to study a carefully chosen set of model organisms that occupy key positions on the tree of life. Just select one from the standard repertoire, and let’s do science! The problem is, however, that the repertoire of established models is not exactly the result of careful deliberation but rather an example of survivorship bias. For example, practical factors, such as amenability to traditional forward genetics, have often determined who got to keep their place in the model zoo. Many of the ‘survivors’ are, understandably, vertebrates, since vertebrates are the ones doing the selection. But vertebrates can be large and cumbersome for microscopy, and some even develop inside the mother, which complicates developmental studies. This often leads people to turn to invertebrates for more basic questions. However, the two most widely used invertebrates both moult the exoskeleton, which makes many aspects of their development fundamentally different from other animals.[5]
Recognizing these limitations and the advances of genome engineering techniques, people began looking for other beasts to populate the empty enclosures in the model zoo. Organisms new and old are gaining traction as models for developmental biology, improving our understanding of common principles, as well as special powers that humans do not possess, such as whole-body regeneration. As already alluded to, I am fascinated by the basic principles of building animal bodies from cells. For studying this, I want an organism with a clearly defined body architecture that is not overly complex. So, what is my fantastic beast of choice? Its name is Nematostella vectensis.
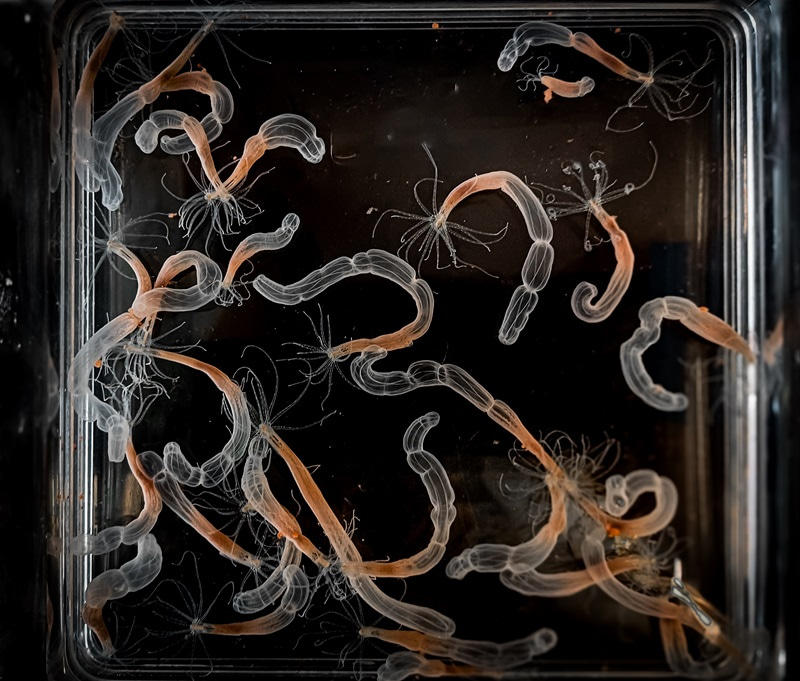
A look inside a box of Nematostella. Photo credit to Jaroslav Ferenc.
Stellas (as we like to call them) might not look fantastic at first sight. When visitors encounter the pale orange tubes resting quietly at the bottom of seawater-filled vessels in the lab, they often ask, “Are they even alive? They’re not doing anything.” But that first sight is deceptive. Stellas are burrowing sea anemones, and much like their more famous Nemo-housing relatives, are fierce predators. In nature, these anemones live in tidal pools burrowed up to the mouth in sand, sticking out a wreath of tentacles ready to catch any small prey unlucky enough to bump into them. Together with corals, jellyfish, and Hydra, Stellas are cnidarians. This means that those tentacles are equipped with venom-injecting stinging cells.
Cnidarians are a sister group to all other animals (bilaterians), and they can provide insights into features of animal development which evolved before the split of these two branches. Sea anemones display the ancestral body plan of cnidarians – the polyp. Most cnidarians spend at least part of their life cycle in this form, essentially a tube with a mouth surrounded by tentacles on one of its ends. Nematostella finishes embryonic development as a 1-millimeter-long primary polyp, already a fully formed multicellular animal.[6] With sufficient feeding, primary polyps will grow in size and sprout additional tentacles, eventually becoming fully-grown adults measuring several centimeters. They are quite hardy, however, and can survive without food for months if necessary. Both primary polyps and adults also regenerate well, being able to regrow missing body parts (or an entire missing half of the body) within just a few days. Despite being a relatively new model, quite a few genetic tools are already available for Nematostella, including RNAi, CRISPR knock-ins and knock-outs, and even inducible expression systems.
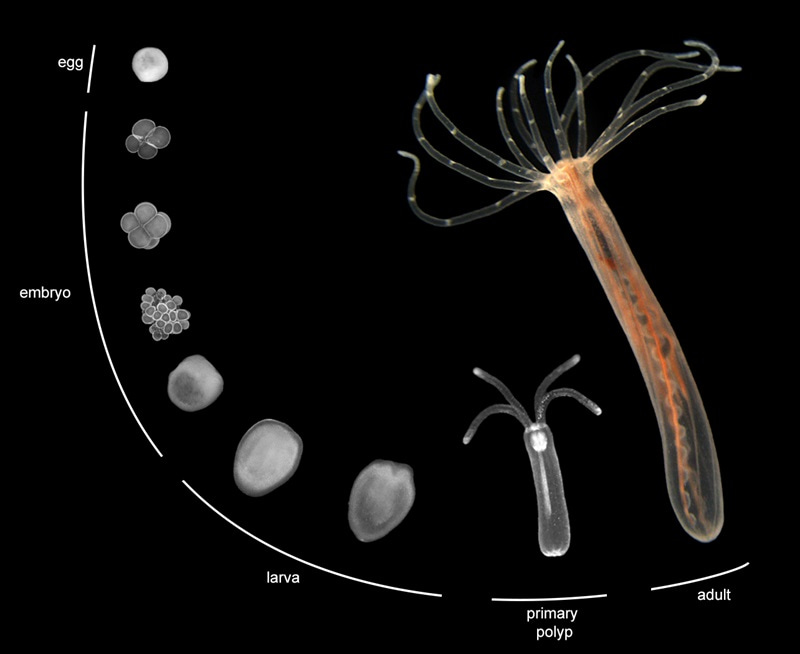
Nematostella life cycle. Stages are not shown to scale. Typical size for embryos and larvae is ~ 200 – 300 μm, for primary polyps 750 μm – 1 mm, and 3-6 cm for adults. Photo credit to Jaroslav Ferenc.
What can Stellas teach us about building and maintaining bodies? I am very curious about the energetics of this process and how the required resources are allocated among different structures.[7] While most tissues and organs are metabolically flexible, some have constrained metabolic preferences – meaning they favor certain fuels regardless of their availability. The most notorious mammalian examples are the brain’s preference for sugars and the heart’s preference for fat as the main energy source. This imposes extra costs, especially during starvation, when other organs often need to compensate by providing a scarce resource. But what might such an arrangement be good for? Current hypotheses conjure explanations connecting the preferences of these “picky eaters” to their function (e.g., brain’s demand for glucose and efficient neurotransmitter synthesis). However, they remain largely untested, and we do not even know whether the evolution of organs like brains and hearts drove the metabolic preferences, or whether they already existed before. This is where our friendly Stellas – which lack brains and hearts, and are very good at starving – offer valuable insights. Surprisingly, they also have structures with restricted fuel preferences, suggesting that such metabolic programs may be an ancient feature that evolved long before the organs displaying them in mammals. Thanks to primary polyps being small enough to be observed as a whole together with all their cells, we are now in a better position to investigate the crosstalk between these structures in vivo and our first results indicate that restricted metabolic programs might have previously unappreciated developmental roles. This line of research will hopefully not only shed light on the functions of restricted fuel preferences but also provide insights into the principles of organismal economy and resource allocation during animal development.
So, should you and everyone else completely abandon traditional models and go chasing after “more fantastic” beasts? Of course not! There is no perfect model organism, and some are better suited to answering certain questions than others. A constructive approach is, however, to not treat research on less established models as if it belonged in a Renaissance cabinet of curiosities or was “not interesting to the broader readership”...

Footnotes
- Ark in Encyclopedia Britannica, 1st edition (1768).
- Darwin’s famous line from “On the Origin of Species”.
- See, for example, Williamson (2009). PNAS, 106(47), 19901-19905. doi.org/10.1073/pnas.0908357106
- This metaphor is inspired by the book ‘The Music of Life’ by Denis Noble.
- For a longer discussion of moulting see for example Valentine & Collins (2000). Evolution & Development, 2(3), 152-156. doi.org/10.1046/j.1525-142x.2000.00043.x
- If you want to learn more about Nematostella beyond what is mentioned here, check Al-Shaer et al. (2021) in Handbook of marine model organisms in experimental biology (pp. 107-128). CRC Press. doi.org/10.1201/9781003217503-7, or Darling et al. (2005). Bioessays, 27(2), 211-221. doi.org/10.1002/bies.20181
- More on the interplay of nutrition and metabolism with development can also be found in Ferenc J, Ikmi A. Nutritional control of developmental processes. Development. 2023 Oct 15;150(20):dev200623. doi.org/10.1242/dev.200623.
Top image of a Nematostella primary polyp with muscles in green, nuclei in magenta, and a subtype of gland cells in yellow. Photo credit to Jaroslav Ferenc.




Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.