Editor's Choice Articles - August 2020

USP15 potentiates NF‐κB activation by differentially stabilizing TAB2 and TAB3
Activation of nuclear factor‐κB (NF‐κB) is a hallmark of inflammation and immunity signalling cascades that must be efficiently regulated to avoid autoimmune disease and chronic inflammation. Now, Zhou and colleagues show that optimal NF‐κB activation after IL‐1β or TNF stimulation is mediated by the deubiquitinating enzyme (DUB) ubiquitin‐specific protease 15 (USP15). 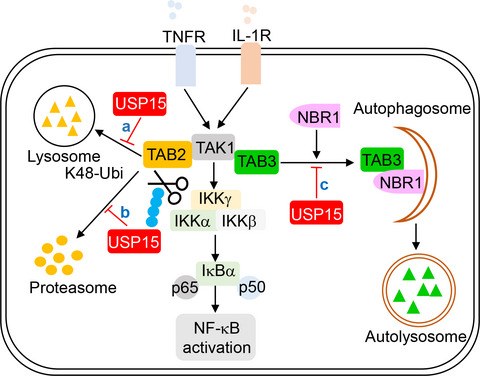
The enzyme targets the TAK1‐TAB complex and increases stability of its subunits TAB1‐3. USP15 cleaves TAB2 ubiquitination but prevents lysosome‐associated degradation of TAB2, as well as selective autophagic TAB3 degradation in a DUB‐independent manner. Together, this work uncovers a mechanism for NF‐κB activation based on TAK1‐TAB complex stabilization.
Read the Original Article here: https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.15202
Read the accompanying commentary here: https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.15210
The adipokine NimrodB5 regulates peripheral hematopoiesis in Drosophila
Growth is regulated by the complex interplay between paracrine and endocrine signals in multicellular organisms. Drosophila larvae provide an ideal model system to explore this interplay, as demonstrated here by Bruno Lemaitre and colleagues.
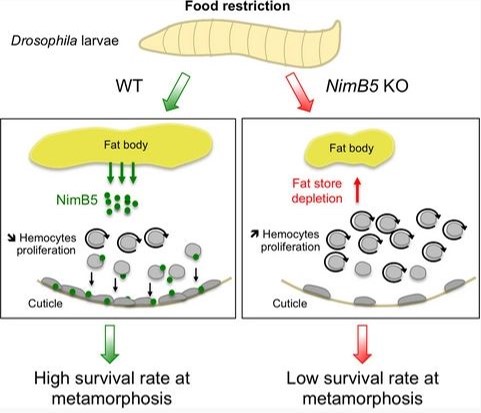 They show that upon starvation, the larval fat body secretes NimrodB5 (NimB5) in the hemolymph and this protein down‐regulates the proliferation and adhesion of hemocytes (blood cells). Blocking this signaling loop results in conditional lethality in larvae raised on a poor diet. This highlights the existence of regulatory mechanisms in animals that allocate resources to the establishment of an immune system depending on nutritional status.
They show that upon starvation, the larval fat body secretes NimrodB5 (NimB5) in the hemolymph and this protein down‐regulates the proliferation and adhesion of hemocytes (blood cells). Blocking this signaling loop results in conditional lethality in larvae raised on a poor diet. This highlights the existence of regulatory mechanisms in animals that allocate resources to the establishment of an immune system depending on nutritional status.
Read the Original Article here: https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.15237
Read the accompanying commentary here: https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.15304





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.