Deamidation as a ticking bomb in proteins: An evolutionarily conserved, yet still enigmatic, mechanism of instability
By Muriel Priault and Joann Kervadec
Enjoy an easy-to-read introduction to deamidation, a post-translational modification that has implications for your favorite protein, pathologies, archaeology, the pharma industry, and more.
Post-translational modifications (PTM) are a very efficient way to implement diversity and produce different proteoforms from a single gene. Over 400 PTM thus expand the array of functions to be executed from an original gene product 1. Twenty proteogenic aminoacids are available to assemble into nascent polypeptides, and in the most common PTMs, a functional group is added on a given residue by enzymatic catalysis. Deamidation stands out in this landscape, and is very unique in more ways than one. This is the playground of our team called Protein Instability and Molecular Aging team (PRIMA) (https://www.ibgc.cnrs.fr/la-recherche/protein-instability-and-molecular-aging/).
A peculiar post-translational modification
Out of the 20 aminoacids available to assemble proteins, there are only two containing an amide group in their lateral chain: Asparagine (Asn) and Glutamine (Gln). Deamidation defines the unrelenting, spontaneous and irreversible destruction of these residues; unlike the vast majority of post-translational modifications, this one does not require any enzyme - it responds to chemistry and time alone (see next paragraph). This implies that unlike any other post-translational modification, this one occurs in living organisms as well as in any protein sample, and anywhere (see the last paragraph).
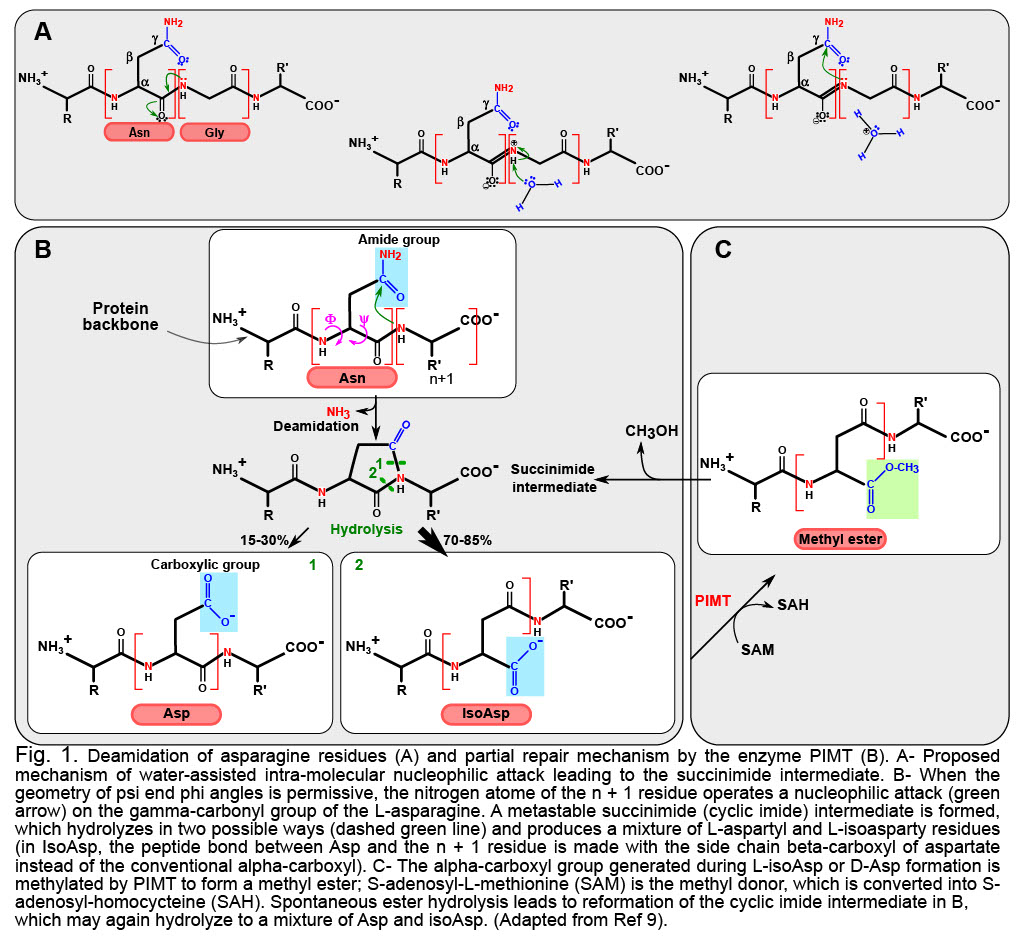
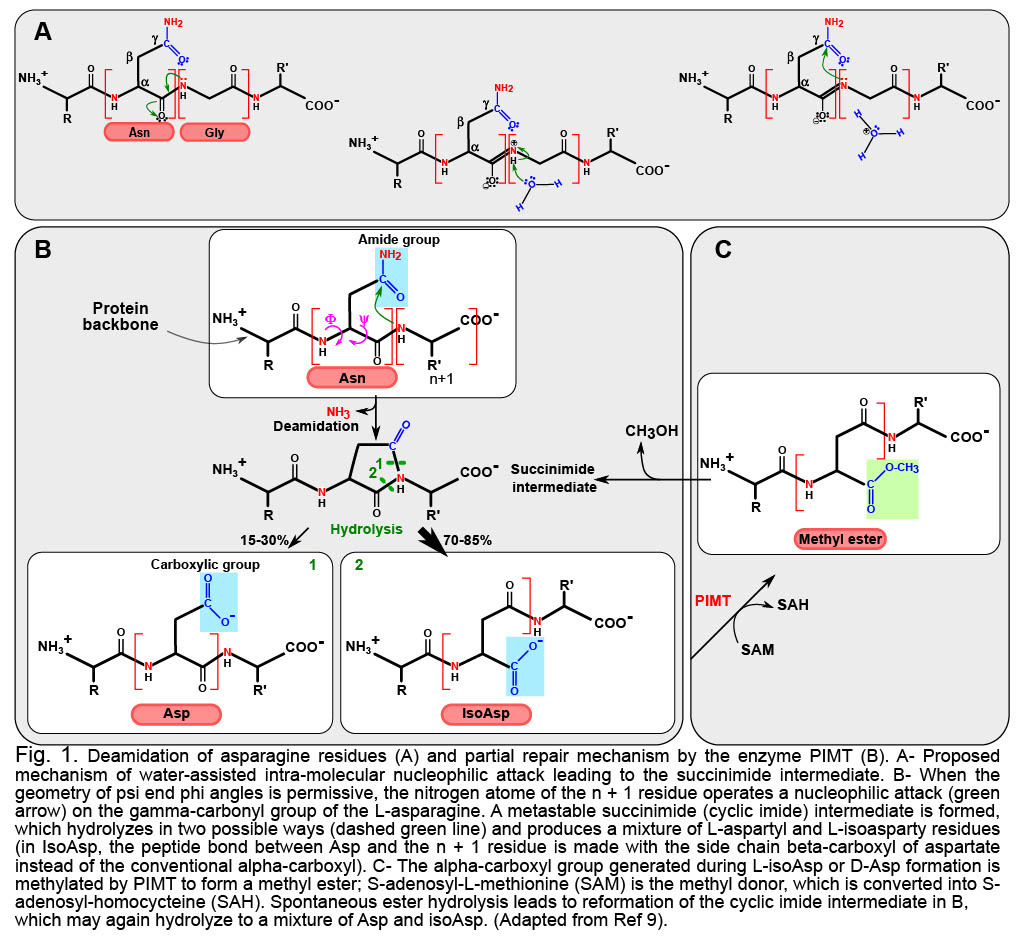
Deamidation is the multi-step reaction (Figure 1A and 1B) that drives the destruction of the amide group in Asn or Gln, leaving instead only a carboxyl group. Asn is thereby converted into Asp, and Gln into Glu. The modification is already noticeable because acidic residues now replace neutral ones, and at physiological pH, the negative charge introduced has the potential to modify the structure/activity/stability of the deamidated product. Yet, if this was all deamidation is about, it would not be overly striking, and it would introduce moderate diversity in the protein encoded. What makes deamidation so remarkable is that most of the time the reaction will not produce the natural Asp, but instead the “non-natural” Iso-Asp. This is because in cell (at pH ~ 7), deamidation proceeds from the formation of a cyclic intermediate that can open by breaking either of two bonds (Figure 1b). Experimental data on peptides indicate that ~30% of the products will be Asp, leaving a large majority of ~70% of Iso-Asp (reviewed in Ref. 2).
“Why does this matter?”, you may ask. Because in an Iso-Asp version of a deamidated protein, the carbon atom that was formerly in beta position of the lateral chain of Asn or Gln is now incorporated in the main chain of the protein. You may disregard this lengthening of the protein main chain, but your immune system will not! Iso-Asp are suspected to drive autoimmune reactions like in type I diabetes where deamidated proteins are categorized as autoantigens setting off the immune-mediated destruction of pancreatic beta cells3. Park et al. further reported that deamidated fibronectin activates monocytic and endothelial cells and promotes atherosclerosis4. Other examples of a much longer list highlight deamidated proteins are markers of neurodegenerative disorders5,6. The only repair mechanism available in mammalians is operated by the protein L-isoaspartate (D-aspartyl) O-methyltransferase PIMT, which very inefficiently repairs IsoAsp into Asp, but does not restore the original gene product7 (Figure 1C).
The final layer of complexity in this reaction is the fact that deamidation also drives L- to D- isomerization when the cyclic intermediate opens, thus further increasing the structural remodeling (reviewed in Ref. 8).
The toll taken by deamidation on our health is just beginning to be unraveled. Innovation potential is very high in this area; if research intensifies and overcomes current limitations, there is little doubt that deamidation will be traced in a growing number of pathologies, that new molecular targets will be identified, and that this exploration will translate into innovative therapeutic strategies.
The question is not if, but when
The former paragraph explained that deamidation is spontaneous, and that this chemical reaction does not need any enzyme to be catalyzed. This paragraph will tell you why you should hurry and look up prediction algorithms to check how fast your favorite protein deamidates (for a review, see Ref. 9)!
Deamidation responds to chemical parameters categorized as intrinsic ones (inherent to the protein) and extrinsic ones (describing the conditions the protein is exposed to).
The speed at which proteins deamidate is actually encoded in their geometry: deamidation proceeds from an intramolecular contortion that is facilitated (i) when the steric hindrance of the n+1 residue is minimal (fastest rates are when the n+1 residue is a Glycine10); (ii) when local secondary structure produces optimal dihedral angles for a suited distance between n+1 nitrogen atom and the side-chain gamma carbon and (iii) when solvent exposure is maximal, allowing access of the water molecule needed to initiate the reaction in an aqueous environment (Figure 1a).
Among extrinsic parameters influencing the catalysis of the reaction, temperature is one of the most tractable ones: to prevent deamidation, samples must be kept at -80 °C, and consistently, catalysis at 37 °C will be faster than at 4 °C. pH is also decisive to determine if deamidation will proceed through direct hydrolysis (below pH 5), or if an imide intermediate will be formed (at pH>7)9. Logically, the deamidation rate is the lowest at pH 7. Extrinsic parameters also encompass the ionic strength of the storage solution, which takes into account the concentration of ions and their charge. But the nature of the ions is a compounding factor in the catalytic effect of the solution. Indeed, phosphate and ammonia are strong catalysts while Tris is not, but all of them impact both pH and ionic strength. Thus, short of tools to predict the composition best suited for the storage of a given protein, its choice is often empirical. Finally, an extrinsic parameter only applying to the cellular context is the half-life of the protein. In living organisms, proteins are built, potentially modified, and finally destroyed when they are obsolete or have accumulated structural damage incompatible with their function. They become susceptible to destruction either by specific proteases, or by the proteasome, or by autophagic processes. Deamidation is a slow reaction, ranging from minutes to days for Asn, and from hundreds of days to centuries for Gln. Logically, proteins with fast cellular turnover will not undergo deamidation.
Regardless, it is estimated that a typical eukaryotic cell with ~10,000 different proteins of 400 residues in average would harbor ~640,000 potential sites of phosphorylation, based on the occurrence of Ser (6.8%) Thr (5.9%) and Tyr (3.2%); by comparison, we previously calculated that the occurrence of Asn (4.3%) and Gln (4.2%) predicts ~400,000 deamidation sites11. Therefore, the question is not if your favorite protein will deamidate, but when!
Deamidation for whom, and where to?
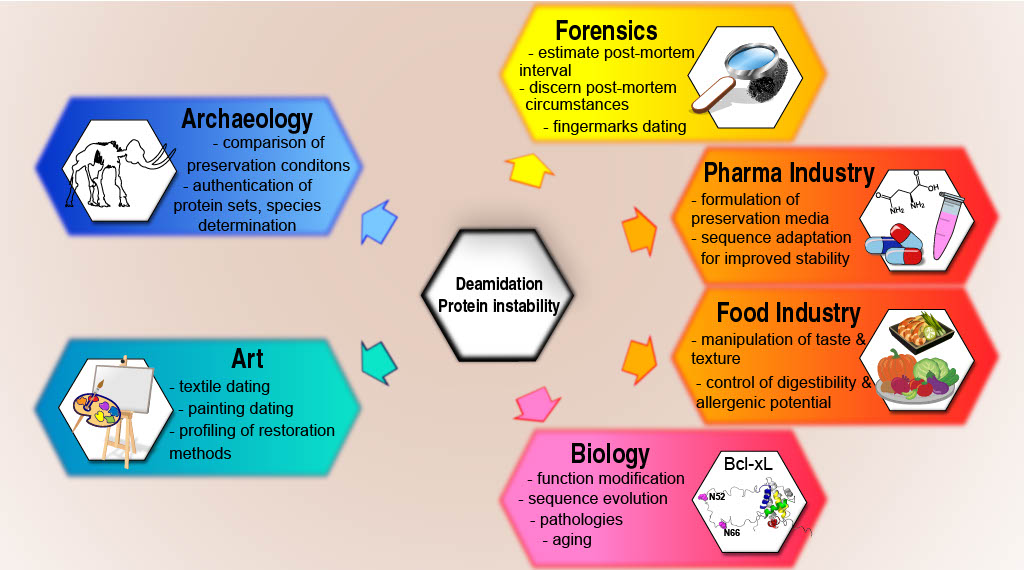
All the above pave the way for this last paragraph, to reflect on who is (or should be) interested in protein deamidation (Figure 2, adapted from Ref. 9), and on the current limitations to overcome and advance the field.
Deamidation is built-in instability, timing the usefulness of proteins in living organisms; the functional loss entailed directly connects to pathology as mentioned above. But this molecular clock also connects with organisms aging, and foreseeable objectives are to identify deamidated proteins as new biomarkers (of specific pathologies or of global aging) to invent efficient therapies against diseases or treatments to age well.
Deamidation also ticks in any protein samples from the past, explaining the interest of archaeologists/paleontologists who study our molecular past: they use deamidation as a dating tool spanning ages > 1 million years while radiocarbon dating stops at ~60 thousand years. Deamidation is a preservation marker of fossils but also of contemporary remains because forensic experts use it to estimate the subject’s age at death or the post-mortem interval for decomposed cadavers.
Deamidation additionally serves to authenticate objects, art works or protein sets by showing if ancient proteins have been contaminated by modern ones. This highlights how precious deamidation is to reconstruct our cultural heritage.
But the deamidation clock starts ticking as soon as a protein is produced, even if in a bioreactor. Hence, in the pharmaceutical industry, the shelf-life and bio-activity of engineered proteins used to cure patients is shortened by deamidation. The stakes here are to tailor production procedures to limit deamidation; it can further be anticipated that computational biology and AI will help predict deamidation hot-spots to harness this molecular decay.
Finally, the food industry shows growing interest in deamidation as a most effective process to modify the physicochemical properties of nutrients for the tuning of taste, texture, or digestion. Besides, deamidation correlates more and more clearly with allergy and inflammatory bowel disease, indicating that the public’s attention will likely focus more and more on deamidation.
What are we missing then? Thanks to research carried out by the different communities mentioned above, deamidation is acknowledged as a most erratic clock, ticking at a different rate at every Asn or Gln residue in every protein. The first challenge to overcome is to be able to predict accurately where and when deamidation will occur in proteins instead of peptides. Also, at the technological level, the 1 Da difference and additional negative charge between a native protein and its deamidated version is currently best detected and quantified by mass spectrometry. However, in complex sets of proteins, determining the extent of deamidation is still not straightforward, as the signs of deamidation are often in the background noise. Reference databases to assign deamidation signatures do not exist for proteins from many still living organisms, let alone from extinct species.
Therefore, more technological evolution to assist deamidation detection/quantification is imperative to push the study of deamidation beyond current obstacles. The way forward is through the intensification of collective work, and the endeavor was recently initiated by a consortium of European scientists who aim to establish a European network dedicated to deamidation.
REFERENCES:
1 Khoury GA, Baliban RC, Floudas CA. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci Rep 2011; 1: 90. https://doi.org/10.1038/srep00090 Link
2 Robinson NE, Robinson AB. Molecular clocks: deamidation of asparaginyl and glutaminyl residues in peptides and proteins. Althouse Press. 2004. ISBN: 1-59087-250-0
3 Wägner AM, Cloos P, Bergholdt R, Boissy P, Andersen TL, Henriksen DB et al. Post-translational protein modifications in type 1 diabetes: a role for the repair enzyme protein-L-isoaspartate (D-aspartate) O-methyltransferase? Diabetologia 2007; 50: 676–681. https://doi.org/10.1007/s00125-006-0556-1 Link
4 Park JE, JebaMercy G, Pazhanchamy K, Guo X, Ngan SC, Liou KCK et al. Aging-induced isoDGR-modified fibronectin activates monocytic and endothelial cells to promote atherosclerosis. Atherosclerosis 2021; 324: 58–68. https://doi.org/10.1016/j.atherosclerosis.2021.03.020 Link
5 Wang J, Zhang Y-R, Shen X-N, Han J, Cui M, Tan L et al. Deamidation-related blood biomarkers show promise for early diagnostics of neurodegeneration. Biomark Res 2022; 10: 1–10. https://doi.org/10.1186/s40364-022-00435-8 Link
6 Ly CV, Ireland MD, Self WK, Bollinger J, Jockel‐Balsarotti J, Herzog H et al. Protein kinetics of superoxide dismutase‐1 in familial and sporadic amyotrophic lateral sclerosis. Ann Clin Transl Neurol 2023; doi : 10: 1012–1024. https://doi.org/10.1002/acn3.51784 Link
7 R. Desrosiers R, Fanelus I. Damaged Proteins Bearing L-Isoaspartyl Residues and Aging: A Dynamic Equilibrium Between Generation of Isomerized Forms and Repair by PIMT. CAS 2011; 4: 8–18. doi : 10.2174/1874609811104010008 https://doi.org/10.2174/1874609811104010008 Link
8 O’Connor C. Protein L-isoaspartyl, D-aspartyl O-methyltransferases: Catalysts for protein repair. In: The Enzymes. 2006, pp 385–433. doi : 10.1016/S1874-6047(06)80015-6 http://dx.doi.org/10.1016/S1874-6047(06)80015-6 Link
9 Boudier-Lemosquet A, Mahler A, Bobo C, Dufossée M, Priault M. Introducing protein deamidation: Landmark discoveries, societal outreach, and tentative priming workflow to address deamidation. Methods 2022; 200: 3–14. doi : 0.1016/j.ymeth.2021.11.012. Link
10 Robinson NE, Robinson AB. Molecular clocks. Proceedings of the National Academy of Sciences of the United States of America 2001; 98: 944. https://doi.org/10.1073/pnas.98.3.944 Link
11 Beaumatin F, El Dhaybi M, Bobo C, Verdier M, Priault M. Bcl-xL deamidation and cancer: Charting the fame trajectories of legitimate child and hidden siblings. Biochim Biophys Acta 2017. doi:10.1016/j.bbamcr.2017.06.012. Link





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.