Creating patient impact through repurposing research

Drug repurposing – utilizing an already approved therapy in a new indication – has been used as a therapeutic strategy for many years. Considering that there are over 7,000 unsolved diseases and more than 20,000 drugs approved by the U.S. Food and Drug Administration (FDA)1 alone, it is no surprise that researchers, clinicians and patients view drug repurposing as a valuable approach to improve patient outcomes and quality of life. In fact, an estimated 20% of all prescriptions in the U.S. are written for an indication other than its approved one,2 a practice known as off-label use.
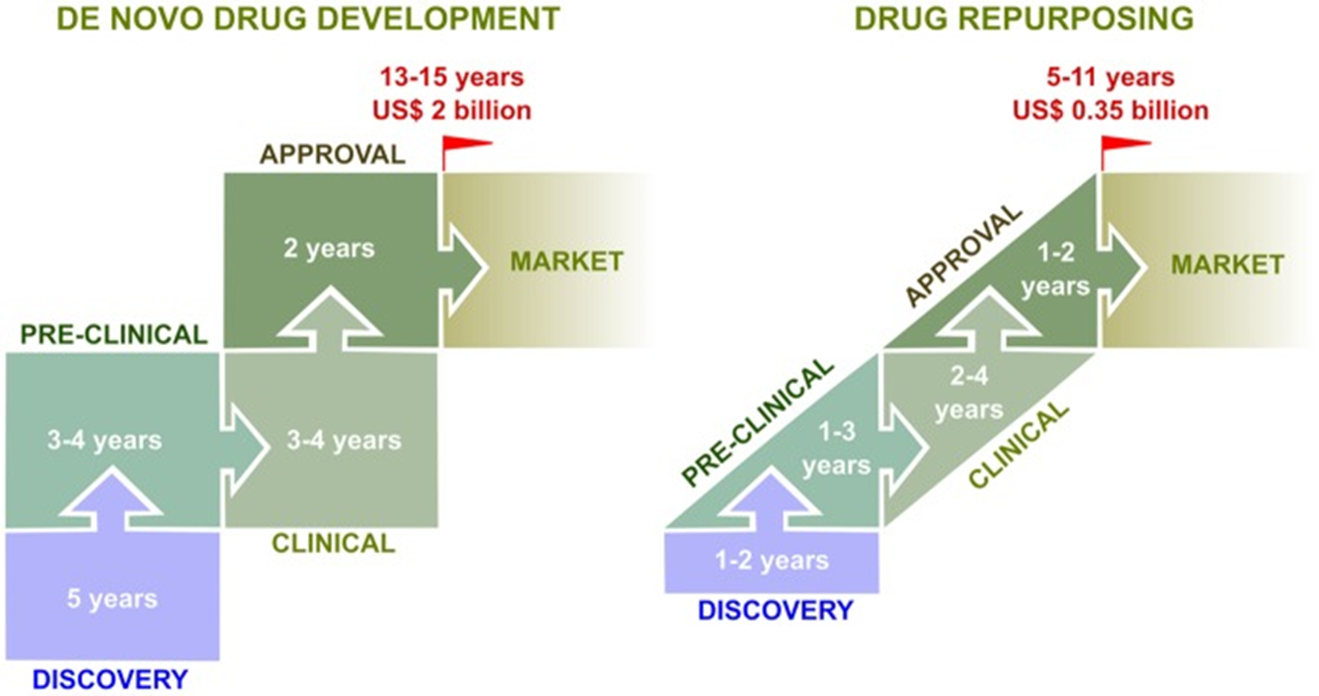
Adapted from Pizzorno A, et al. Front. Immunol., 19 March 2019. https://doi.org/10.3389/fimmu.2019.00531
As well-known as drug repurposing is in the medical world, its benefits of speed, safety and cost-effectiveness have not always been as obvious to the general public. Some people may know that aspirin, a drug that has been around for more than a century as a pain reliever, is also used to help protect certain patients from heart attack or stroke.3 Yet they may not realize that Botox was originally developed to treat a rare eye muscle condition and has since been repurposed in cosmetic procedures to migraines to overactive bladder.4 Or that the drug thalidomide, which had devastating effects for pregnant women and their babies in the 1950s and 1960s, found new life as an effective treatment for the rare cancer multiple myeloma.5
However, the COVID-19 pandemic has greatly increased the general public’s awareness and understanding of repurposing. Over the past two years, many non-scientific news outlets have carried stories focusing on repurposing clinical trials to treat COVID-19. And with good reason. As of this writing, the World Health Organization (WHO) recommends using the already approved and available drugs dexamethasone, baricitinib and tocilzumab to treat the sickest COVID-19 patients, and the WHO is continuing to evaluate other repurposed drugs as possible COVID-19 treatments, alongside de novo therapies.6
Cures Within Reach (CWR) is a not-for-profit leader in leveraging the speed, safety and cost-effectiveness of testing already approved therapies, including drugs, devices and nutraceuticals, to address unmet medical needs. For nearly 15 years, CWR has been finding and funding early-stage clinical repurposing trials to catalyze follow-on trials that may lead to a change in clinical practice. CWR has funded over 100 repurposing trials at more than 60 different institutions worldwide. CWR is disease-agnostic, geography-agnostic and institution-agnostic, as well as indifferent to the commercial value of its funded trials. CWR’s funding is often instrumental in bridging the gap between preclinical studies and larger clinical trials. In fact, the $7.5 million invested in repurposing research by CWR to date has been leveraged into over $75 million in follow-on funding by our researchers.

The clinical trials funded by CWR are built on preclinical evidence supporting the repurposing idea. Historically, much of the preclinical evidence was developed using cell, tissue or animal models. One of CWR’s earliest success stories was in the rare pediatric blood disorder autoimmune lymphoproliferative syndrome (ALPS). With support from CWR, Dr. David Teachey at the Children’s Hospital of Philadelphia researched the potential of the immunosuppressive generic drug sirolimus to treat ALPS. Dr. Teachey first conducted a successful mouse study,7 and the data from this study were critical in designing a follow-on clinical trial. This pivotal trial showed that sirolimus could be an effective treatment for ALPS, with nearly all patients experiencing a partial or complete remission.8 Today, sirolimus is considered a standard of care for ALPS patients.
Technological advances have allowed bioinformatics, machine learning and artificial intelligence to become effective preclinical tools in generating repurposing evidence. Recently, researchers at Massachusetts General Hospital and Harvard Medical School published a machine learning framework called Drug Repurposing in Alzheimer’s Disease (DRIAD). DRIAD can analyze large datasets to learn and identify approved drugs that may be effective in treating Alzheimer’s disease.9 As one of the study’s authors stated, DRIAD can be used for “prioritizing drugs, helping clinical studies to focus on the most promising ones.”10
Preclinical research can also advance drug repurposing by reformulating an existing drug for a new method of delivery, such as turning an oral drug into an injectable. Reformulations can be important for two main reasons. First, changing a repurposed drug’s delivery method may create broader patient impact in a wider range of diseases. In addition, reformulation may result in intellectual property and commercial opportunities. Researchers at the University of Kansas Cancer Center caught the attention of healthcare-focused investors when they discovered fosciclopirox, the prodrug of the topical generic antifungal drug ciclopirox with known anticancer properties. CicloMed, a U.S. based company, is now developing an intravenous form of fosciclopirox in bladder cancer and acute myeloid leukemia, improving the drug’s ability to treat cancer.11, 12
Identifying and characterizing combinations of approved drugs that may have a synergistic effect is another important way preclinical research influences clinical repurposing trials. CWR is funding a canine comparative trial at the University of Cambridge, treating naturally occurring bone cancer in dogs. Post-surgical inflammation has been implicated in promoting cancer metastasis, and Professor Matthew Allen is combining two non-oncology drugs, propranolol and robenacoxib, in osteosarcoma to reduce this inflammation. Although both drugs have known anti-inflammatory effects, the combination may have a greater impact that either by itself.13 Data from this ongoing study could provide a strong rationale for repurposing clinical trials in human osteosarcoma patients. A repurposed therapy can also be combined with the standard of care, as in a CWR-funded clinical trial in Meniere’s disease. Led by Dr. Michael Strupp at the Hospital of the University of Munich, this trial is investigating the ability of selegiline, approved for Parkinson’s disease, to improve the clinical efficacy of betahistine, already approved in Europe to treat Meniere’s disease. The addition of selegiline may also allow clinicians to treat patients with a lower dose of betahistine than currently recommended.
The above examples show how preclinical scientists and their work are integral to successful clinical repurposing trials. If you are interested in adding a repurposing strategy to your research, CWR suggests starting with the following questions:
- What resources at your institution or other institutions can help advance your repurposing idea? As interest in translational research has grown over the past several years, many institutions have added core facilities or centers with repurposing resources. For example, University College London (UCL) is developing a Therapeutic Innovation Network specifically for drug repurposing,14 and the Broad Institute in Boston, Massachusetts created the Drug Repurposing Hub as an open-source drug screening library including approved therapies.15 In addition, a new European consortium, RePo4EU, was recently founded to leverage artificial intelligence in developing a platform to support drug repurposing at any stage of the research pipeline.16
- Who else at your institution or in your network is focused on repurposing? Learning from others about what has or hasn’t worked for them is a great way to start your repurposing career, and colleagues can be a valuable resource for repurposing best practices. You might even find new collaborators from outside your department or discipline, with whom you might not typically work.
- Who might be interested in taking your research to the next step? Connecting early on with researchers and clinicians who can advance your work informs the design of your research and creates in more actionable data. Keeping clinical care and patients in mind while thinking about the next step (or beyond) will also give your research greater impact.
- What funding sources are available to support your research? CWR posts our open funding opportunities for repurposing trials on our website, and we encourage cross-border collaborations, such as an ongoing brain cancer trial with a U.S./Netherlands collaboration. CWR is certainly not alone in funding repurposing research. Other nonprofit organizations, such as the Alzheimer’s Drug Discovery Foundation17, Anticancer Fund18 and LifeArc19 also fund repurposing research as a therapeutic development strategy.
To learn more about repurposing and the work of Cures Within Reach, please contact Clare Thibodeaux, PhD at [email protected].
References:
- https://www.fda.gov/about-fda/fda-basics/fact-sheet-fda-glance
- David C Radley 1, Stan N Finkelstein, Randall S Stafford. Arch Intern Med. 2006 May 8;166(9):1021-6. doi: 10.1001/archinte.166.9.1021.
- Andrew T. Peters, MD, R. Kannan Mutharasan, MD. JAMA. 2020;323(7):676. doi:10.1001/jama.2019.18425
- Ram Kumar Dhaked, Manglesh Kumar Singh, Padma Singh, Pallavi Gupta. Indian J Med Res. 2010 Nov; 132(5): 489–503.
- Waqas Rehman, Lisa M. Arfons, Hillard M. Lazarus. Ther Adv Hematol. 2011 Oct; 2(5): 291–308.
- https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.2
- David T. Teachey, Dana A. Obzut, Kelly Axsom, John K. Choi, Kelly C. Goldsmith, Junior Hall, Jessica Hulitt, Catherine S. Manno, John M. Maris, Nicholas Rhodin, Kathleen E. Sullivan, Valerie I. Brown, Stephan A. Grupp. Blood. 2006 Sep 15; 108(6): 1965–1971.
- David T Teachey, Robert Greiner, Alix Seif, Edward Attiyeh, Jack Bleesing, John Choi, Catherine Manno, Eric Rappaport, Dirk Schwabe, Cecilia Sheen, Kathleen E Sullivan, Hongming Zhuang, Daniel S Wechsler, Stephan A Grupp. Br J Haematol. 2009 Apr;145(1):101-6.
- Steve Rodriguez, Clemens Hug, Petar Todorov, Nienke Moret, Sarah A. Boswell, Kyle Evans, George Zhou, Nathan T. Johnson, Bradley T. Hyman, Peter K. Sorger, Mark W. Albers, Artem Sokolov. Nat Commun 12, 1033 (2021).
- https://www.massgeneral.org/news/press-release/Artificial-intelligence-reveals-current-drugs-that-may-help-combat-alzheimers-disease
- https://www.ciclomed.com/lead-product
- https://www.businesswire.com/news/home/20211201005405/en/Notable-and-CicloMed-Initiate-Phase-1B2A-Clinical-Trial-of-Fosciclopirox-in-Acute-Myelogenous-Leukemia-Under-Co-Development-Agreement
- Sorski Liat, Melamed Rivka, Matzner Pini, Lavon Hagar, Shaashua Lee, Rosenne Ella, Ben-Eliyahu Shamgar. Brain Behav Immun. 2016 Nov; 58: 91–98.
- https://www.ucl.ac.uk/therapeutic-innovation-networks/about/our-networks
- https://clue.io/repurposing
- https://repo4.eu/
- https://www.alzdiscovery.org/
- https://www.anticancerfund.org/
- https://www.lifearc.org/
Photo by National Cancer Institute on Unsplash





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.