February - Issue 3
Identification of a novel botulinum neurotoxin gene cluster in Enterococcus
Peck, M.W. and colleagues, FEBS Letters
Clostridium botulinum neurotoxins (BoNTs) cause fatal neuroparalysis (botulism) by targeting the SNARE docking proteins of cholinergic neurons, thereby preventing exocytosis of the neurotransmitter acetylcholine. Here, using bioinformatics, the authors report the first complete boNT gene cluster in a non-clostridial genome. Identified in a species of Enterococcus, the putative BoNT contains all the functional domains of a typical BoNT and a novel cell attachment domain that has implications for pharmaceutical applications.
Single-molecule nucleosome remodeling by INO80 and effects of histone tails
Michaelis, J. and colleagues, FEBS Letters
INO80 is a multi-subunit ATP-dependent chromatin remodeling complex that functions in DNA replication, transcription, and damage repair. Here, the authors develop a single-molecule Foerster Resonance Energy Transfer (FRET) assay to investigate nucleosome sliding and positioning by INO80 and the influence of histone tails on this process. The findings indicate that histone tails pose a major regulatory barrier for the initiation of nucleosome remodeling by INO80.
Reversible optical control of F1F0-ATP synthase using photoswitchable inhibitors
Trauner, D. and colleagues, FEBS Letters
The F1Fo-ATP synthase is a macromolecular machine that functions via a reversible rotary mechanism to synthesize and hydrolyze ATP. Here, the authors synthesize polyphenol derivatives that undergo photoisomerization and bind ATP synthase in their trans, but not cis form. These photoswitches are shown to reversibly inhibit the ATPase activity of the yeast Yarrowia lipolytica native ATP synthase. The study paves way for spatiotemporal control of ATP synthase activity by light.
A candidate functional SNP rs7074440 in TCF7L2 alters gene expression through c-FOS n hepatocytes
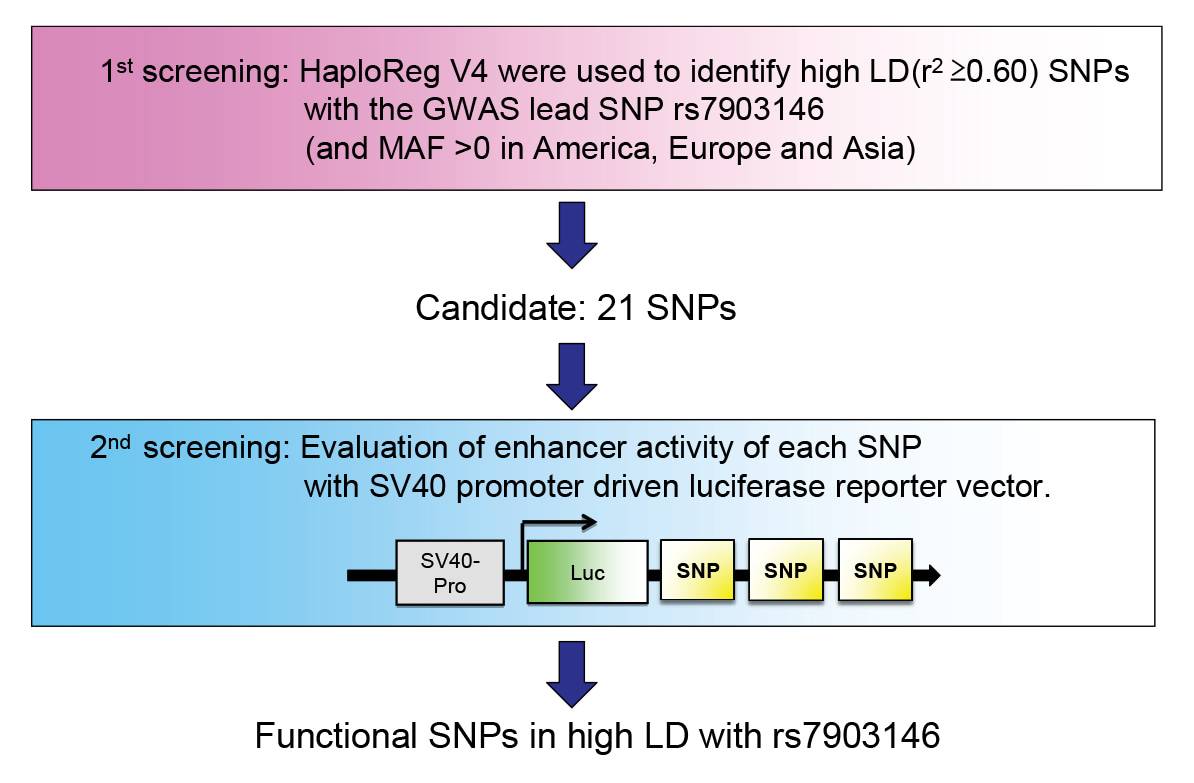
Shimano, H. and colleagues, FEBS Letters
Transcription factor 7-like 2 (TCF7L2) is involved in glucose metabolism, and single nucleotide polymorphisms (SNPs) within TCF7L2 are the most strongly associated genetic markers of type 2 diabetes. The mechanisms through which SNPs regulate TCF7L2 expression remain elusive. Here, the authors identify a novel functional SNP that enhances TCF7L2 expression in hepatocytes and show that it regulates TCF7L2 expression through direct interaction with the transcription factor c-FOS.
February - Issue 4
ARRDC3 suppresses colorectal cancer progression through destabilizing the oncoprotein YAP
Zhou, Z. and colleagues, FEBS Letters
Arrestin-related domain-containing protein-3 (ARRDC3) promotes lysosome-mediated protein degradation. ARRDC3 expression is downregulated in colorectal cancer (CRC) cells. Shen et al. show that ARRDC3 suppresses colorectal tumorigenesis by binding to and promoting the degradation of YAP, the transcriptional cofactor of the Hippo signaling pathway. Furthermore, overexpression of ARRDC3 neutralizes the resistance of CRC cells towards chemotherapeutic drugs, making ARRDC3 a potential drug target in CRC.
Electrical pulse stimulation induces GLUT4 translocation in a Rac-Akt-dependent manner in C2C12 myotubes
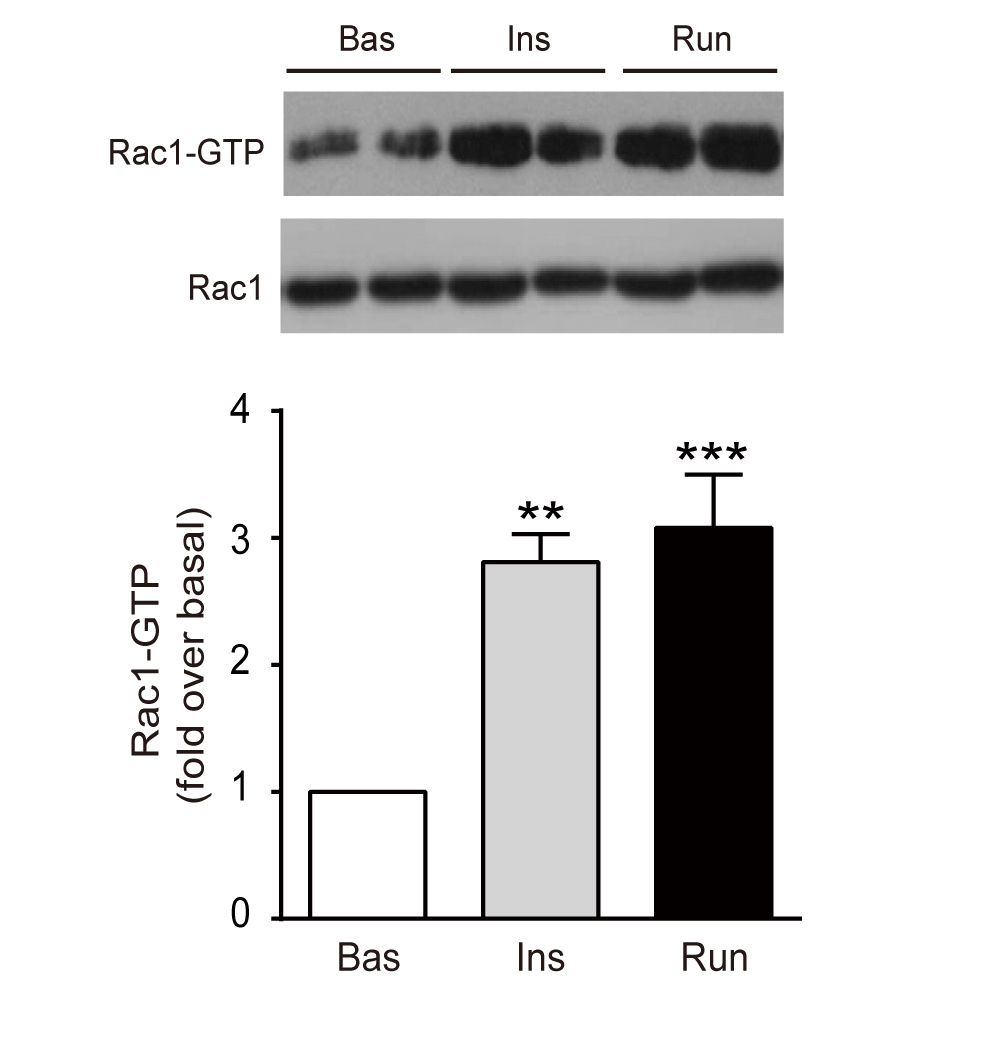
Niu, W. and colleagues, FEBS Letters
Insulin and exercise/muscle contraction stimulate glucose uptake in skeletal muscle, which requires translocation of glucose transporter 4 (GLUT4) from intracellular storage vesicles to the plasma membrane. Here, using electrical pulse stimulation (EPS) to contract C2C12 myotubes, Nui and colleagues provide insights into contraction-stimulated GLUT4 translocation. The Rac1 GTPase and the protein kinase Akt are shown to mediate GLUT4 translocation during skeletal muscle contraction, with Rac1 acting upstream of Akt.
Modulation of GSK3β autoinhibition by Thr-7 and Thr-8
Fu, Z. and colleagues, FEBS Letters
Glycogen synthase kinase 3β (GSK3β) regulates diverse intracellular signaling pathways. Upon insulin stimulation, AKT phosphorylates GSK3β at serine 9 (Ser-9), leading to its autoinhibition. Fu and colleagues find that threonine 7 (Thr-7) and Thr-8, highly conserved residues in the AKT substrate consensus sequence of GSK3β, are essential for AKT-mediated Ser-9 phosphorylation in vivo. The study reveals that the intestinal cell kinase (ICK) phosphorylates GSK3β at Thr-7 and that Thr-7 phosphorlyation blocks GSK3β autoinhibition.


Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.