Highlights from The FEBS Journal - August 2017
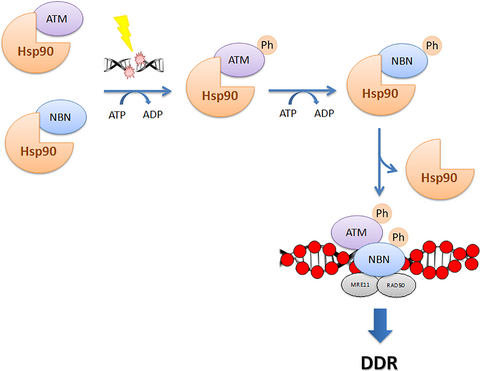
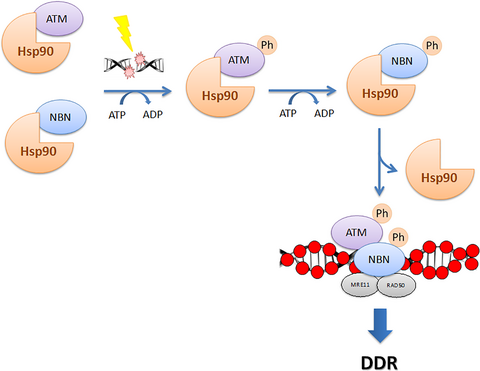
The heat shock protein 90 (Hsp90α) modulates the stability of several DNA damage response (DDR) proteins including MRE11/RAD50/NBN (MRN trimer). di Masi and colleagues now reveal that NBN and the ataxia-telangiectasia-mutated (ATM) kinase are Hsp90α clients and that Hsp90α facilitates NBN–ATM crosstalk in response to DNA damage. Upon IR-induced DNA double-strand breaks (DSBs), ATM phosphorylates NBN and Hsp90α. NBN then dissociates from Hsp90α and relocalises to the DSBs together with MRE11 and RAD50 to promote the DDR.
Read more...
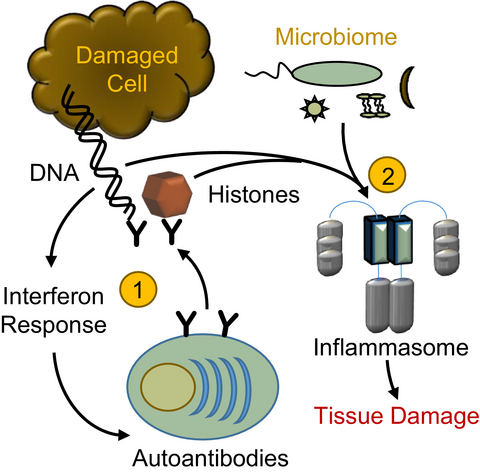
The inflammasome is a protein complex that forms in the cytoplasm in response to cellular damage or the presence of pathogen-associated molecules resulting from infection. Undegraded self-DNA and histones can also induce inflammasome formation and drive ‘sterile inflammation’. In this Viewpoint, Kanneganti and colleagues discuss the roles of the inflammasome during nucleic-acid- and histone-driven ‘sterile inflammation’ and highlight emerging roles of pathogens and commensal microbes in the development of ‘sterile inflammation’.
Read more...





Join the FEBS Network today
Joining the FEBS Network’s molecular life sciences community enables you to access special content on the site, present your profile, 'follow' contributors, 'comment' on and 'like' content, post your own content, and set up a tailored email digest for updates.